Figure 1.
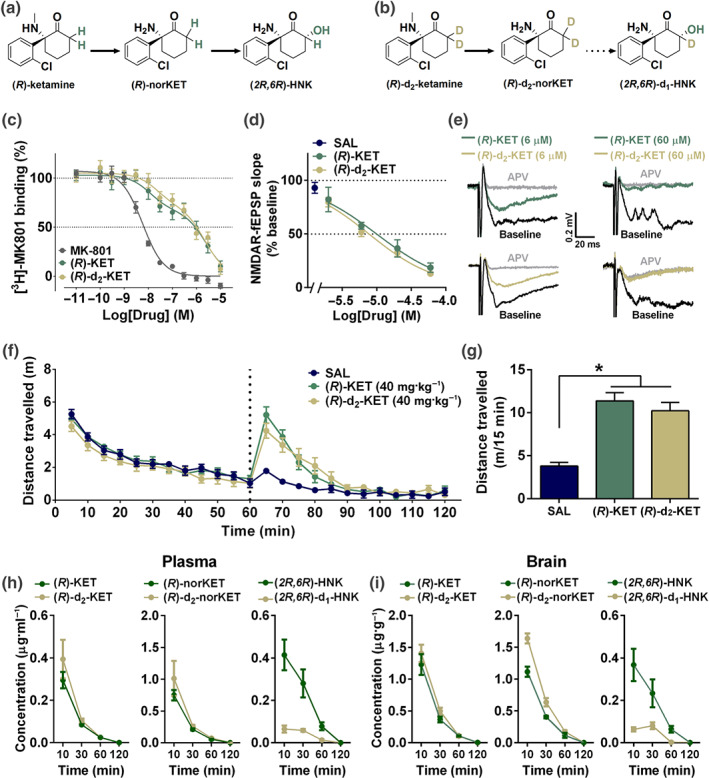
C6‐deuterated (R)‐ketamine attenuates metabolism to (2R,6R)‐hydroxynorketamine without modifying NMDA receptor binding or functional inhibition properties. Metabolic transformations of (a) (R)‐ketamine (KET) and its (b) deuterated isotope at the C6 position, (R)‐d2‐KET. (R)‐KET is demethylated to give (R)‐norketamine (norKET), which in turn is hydroxylated to result in (2R,6R)‐hydroxynorketamine (HNK). (R)‐d2‐KET is hydroxylated to (R)‐d2‐norKET. (R)‐d2‐norKET cannot readily be transformed to (2R,6R)‐d1‐HNK. (c) Both (R)‐KET and (R)‐d2‐KET displace [3H]MK‐801 binding with similar affinity. (d) Concentration–response relationships of NMDA receptor‐mediated fEPSP slope as a function of compound concentration for (R)‐KET and (R)‐d2‐KET. (e) Typical traces of NMDA receptor‐mediated fEPSPs in slices from mouse CA1 stratum radiatum in response to stimulation of the Schaffer collateral axons before and after perfusion of either 6‐ or 60‐μM (R)‐KET and (R)‐d2‐KET. (f) After recording baseline activity for 60 min, mice received drug (vertical dashed line), and locomotor activity was monitored for another 60 min. (g) Total distance moved during the first 15 min following drug exposure. (R)‐KET and (R)‐d2‐KET induced equivalent hyperlocomotor responses at the dose of 10 mg·kg−1. (h) Plasma and (i) brain levels of KET, norKET, and HNK following intraperitoneal administration of (R)‐KET or (R)‐d2‐KET (10 mg·kg−1) in mice. Data are the mean ± SEM. *P < .05, significantly different as indicated. See Tables 2 and S1 for statistical analyses and precise group sizes
