Figure 6. Anchor of PPM1H is a folding motif.
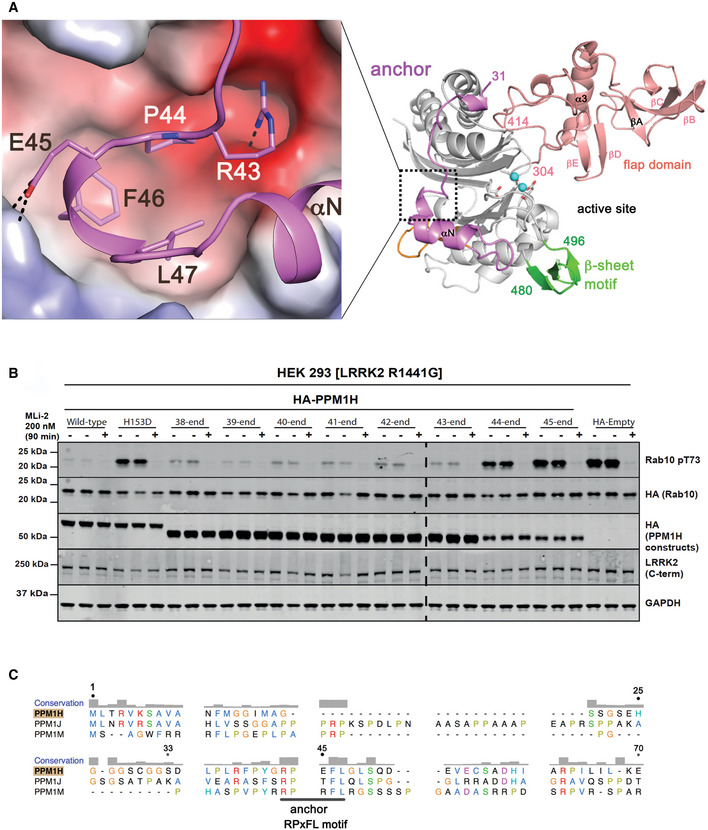
- Interactions between the anchors of PPM1H against the electrostatic surface of the core catalytic domain. The region is placed in context with the dotted box on a ribbon model of PPM1H (right).
- Incremental deletions of the N‐terminal 37 to 44 residues, one residue at a time. Upon deletion of R43 (44‐end), a reduced level of soluble PPM1H expression has no significant catalytic activity.
- Sequence alignment of the N‐terminal regions of the evolutionarily related PPM1H/J/M enzymes. The degree of conservation is above the alignment, and residue numbers correspond to PPM1H. A conserved anchor motif (RPxFL) is annotated below the sequences.
