Figure 8.
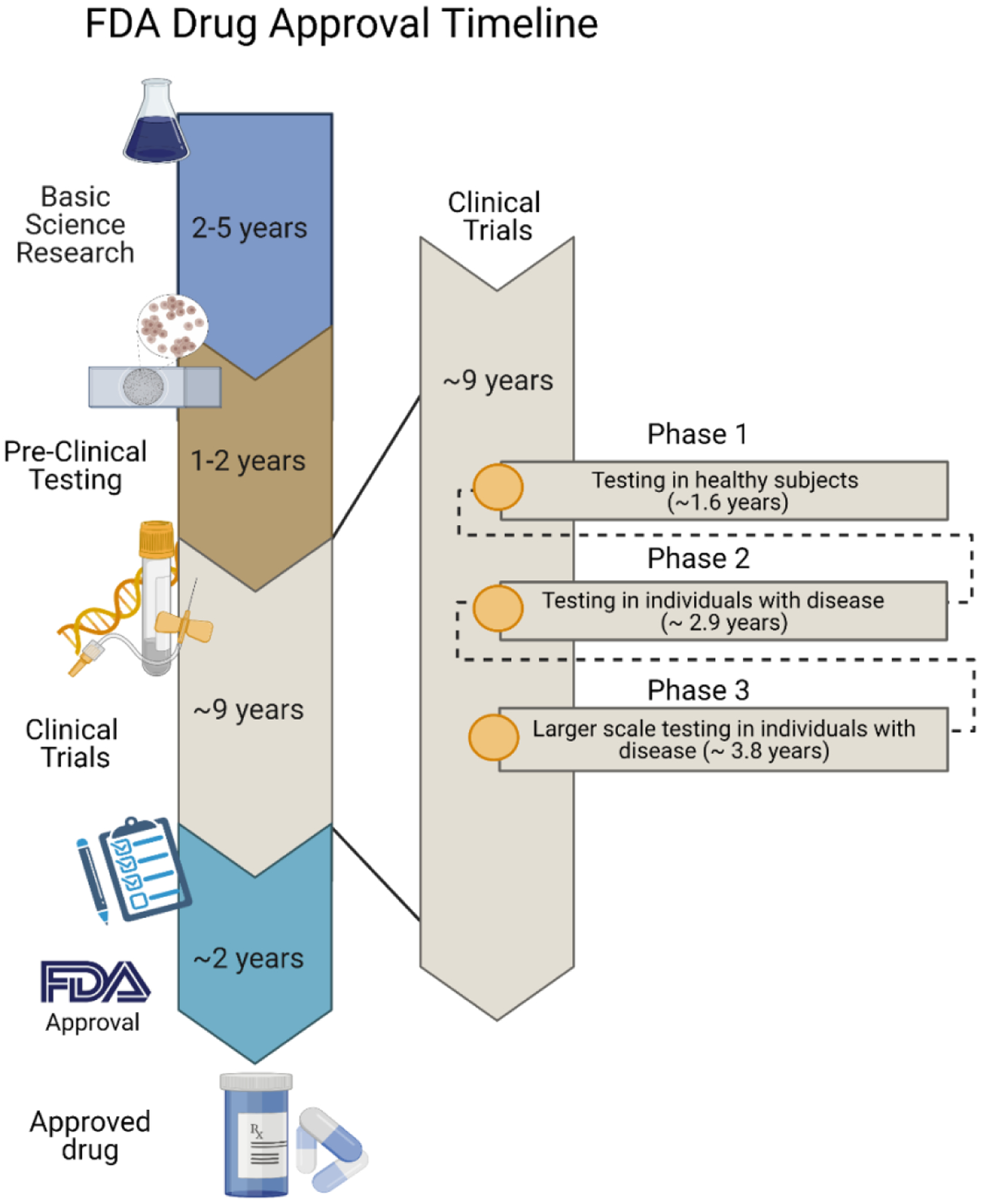
Approximate timelines for FDA drug approval. Based on information in ref. [212]. The average time spent on basic research is 2–5 years followed by 1–2 years of advanced clinical testing and nearly a decade of clinical trials. Average times for each stage of clinical trials are listed in the inset. Finally, the FDA approval process takes roughly 2 additional years. Schematic was made using BioRender.com
