Figure 9.
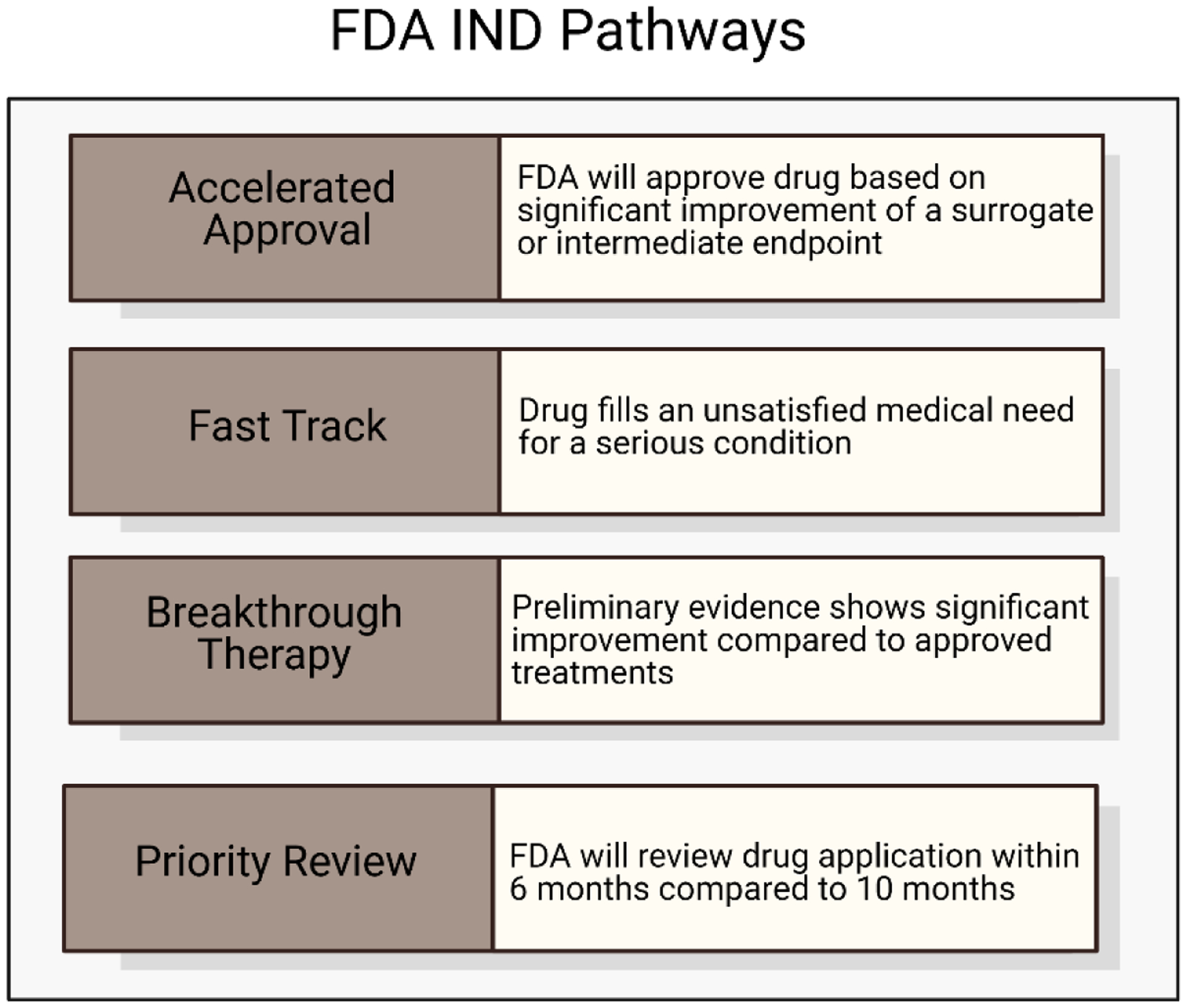
FDA IND Pathways. Four potential pathways for new drug products to be expedited during the FDA approval process. These include accelerated approval, fast track designation, breakthrough therapy designation, and priority review. Adapted from ref. [217]. Schematic was made using BioRender.com
