Figure 3.
Functions of PFDNs in budding yeast
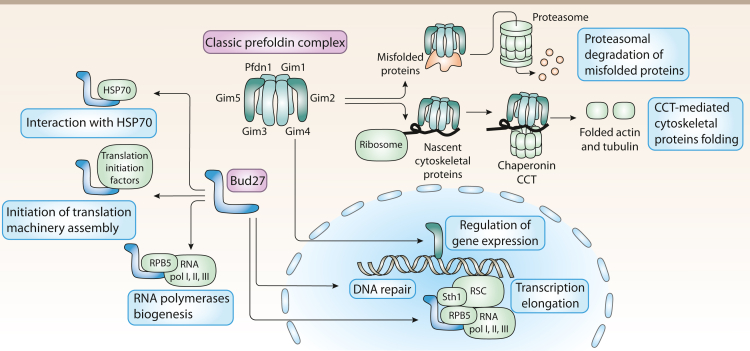
In budding yeast, the classic PFDN complex is formed by proteins Gim1 to Gim 5 and PFDN1. This complex binds to nascent cytoskeletal proteins, such as actin and tubulin, to deliver them to the chaperonin CCT where they complete their folding. It also binds to misfolded proteins to promote their degradation through the delivery to the proteasome machinery. Some components of the complex are also involved in nuclear regulation of gene expression. Yeast presents an atypical PFDN termed Bud27 which is not forming part of any PFDN complex in these organisms. Bud27 also contributes to protein folding by binding to HSP70. It also contributes to the biogenesis of the RNA polymerases I, II, and III thanks to its interaction with RPB5, a common component of the three of them; and to the assembly of the initiation of translation machinery. In the nucleus, Bud27 contributes to transcription elongation by interacting with the polymerases and with chromatin remodelling complexes such as RSC. Loss of URI also increases DNA damage.