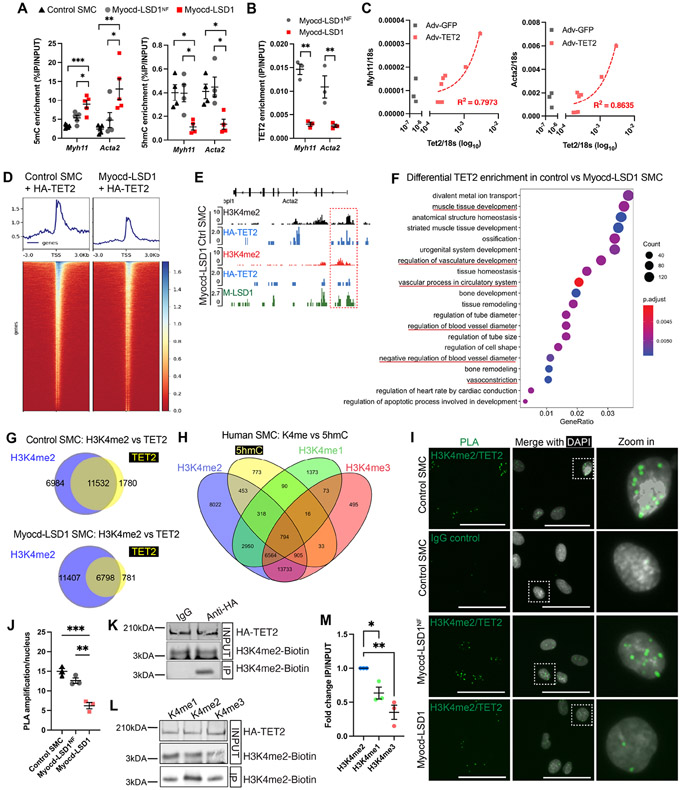
Figure 4: H3K4me2 interacts with TET2 and is required for its recruitment on the SMC contractile genes.
A. 5mC and 5hmC levels on Myh11 and Acta2 CArG box regions in control, Myocd-LSD1, and Myocd-LSD1NF SMC. B. TET2 enrichment on Acta2 and Myh11 CArG box regions in Myocd-LSD1NF and Myocd-LSD1 SMC. C. Correlation between Myh11, Acta2 and Tet2 mRNA levels in Myocd-LSD1 SMC transduced with Adv-TET2. R2 is calculated by linear regression. D. Heatmap of HA-TET2 enrichment around transcription start sites (TSS) measured by CUT&Tag sequencing in control SMC and Myocd-LSD1 SMC transduced with Adv-HA-TET2. E. Visualization of HA-TET2 enrichment peaks on the Acta2 gene in control SMC and Myocd-LSD1 SMC. F. GO pathway analysis of genes with decreased TET2 enrichment in Myocd-LSD1. G. Venn diagrams of annotated genes with HA-TET2 enrichment and H3K4me2 enrichment in control SMC or Myocd-LSD1 SMC. H. Venn Diagram comparing H3K4me1, H3K4me2, H3K4me3, and 5hmC distribution in human SMC. I. Proximity Ligation Assay (PLA) detecting proximity between H3K4me2 and TET2. Scale bar = 25μm. J. Quantification of H3K4me2/TET2 PLA signals in control SMC, Myocd-LSD1NF, and Myocd-LSD1 SMC. K. HA-tagged TET2 and biotin-H3K4me2 peptide co-immunoprecipitation (IP). IP: anti-HA antibody or IgG control; Blotting: anti-biotin antibody. L. HA-tagged TET2 and biotin-H3K4me1/2/3 peptide co-immunoprecipitation. IP: anti-HA antibody; Blotting: anti-biotin antibody. M. Quantification of HA-TET2 and Biotin-H3K4me1/2/3 peptide co-immunoprecipitation. IP signals normalized with INPUT Biotin-H3K4me1/2/3 signals. Data are represented as mean ± s.e.m of 3-5 independent experiments. Groups were compared by One-Way ANOVA. * p<0.05, ** p<0.001, *** p<0.0001.