Abstract
Aging is the major risk factor for neurodegenerative diseases and affects neurite distributions throughout the brain, yet underlying neurobiological mechanisms remain unclear. Multi-shell diffusion-weighted imaging and neurite orientation dispersion and density imaging (NODDI) now provide in vivo biophysical measurements that explain these biological processes in the cortex and white matter. In this study, neurite distributions were evaluated in the cortex and white matter in healthy older adults and patients with amnestic mild cognitive impairment (aMCI) that provides fundamental contributions regarding healthy aging and neurodegeneration. Older age was associated with reduced neurite density and neurite orientation dispersion (ODI) in widespread cortical regions. In contrast, increased ODI was only observed in the right thalamus and hippocampus with age. For the first time, we also reported a widespread age-associated decrease in neurite density along major white matter tracts correlated with decreased cortical neurite density in the tract endpoints in healthy older adults. We further examined alterations in cortical and white matter neurite microstructures in aMCI patients and found significant neurite morphology deficits in memory networks correlated with memory performance. Our findings indicate that neurite parameters provide valuable information regarding cortical and white matter microstructure and complement myeloarchitectural information in healthy aging and aMCI.
Keywords: Alzheimer’s disease, diffusion weighted imaging, healthy aging, neurite morphology
Introduction
Neurons are embedded in long-range and highly complex neural circuits that enable them to receive, process properly, and send various types of information through their dendrite arbors. Genetic and postmortem studies have demonstrated that disruptions to dendritic branching can precipitate neurological aging and neurodegenerative disorders. These disruptions include abnormalities in protein expression supporting neurite and synaptic integrity and myelination (Dickstein et al. 2013; Colgan et al. 2016). However, there are significant barriers to translating these findings into clinical practice. Recent advances in diffusion-weighted magnetic resonance imaging (dMRI) have provided novel approaches in acquisition and modeling to facilitate interrogation of neural microstructure in gray matter and white matter. These new methods may provide more sensitive information regarding healthy aging and neurodegeneration before forming irreversible tissue loss.
New dMRI techniques have facilitated in vivo examination of brain tissue microstructure through modeling the diffusion properties of water molecules within neurites (axons and dendrites) and the extracellular space. While several plausible biophysical models exist, NODDI (the neurite orientation dispersion and density imaging) is a uniquely promising technique for use in clinical applications. In white matter, unlike conventional mathematical models such as the tensor model (DTI), NODDI attempts to separate tissue classes and models geometric representations that partially recapitulate the microstructural environment. In particular, the NODDI model accounts for free-water contamination that is particularly relevant to understanding aging and neurodegeneration processes in the brain (Rathi et al. 2014). Accordingly, recent studies have demonstrated that NODDI indices have overcome the limitations of conventional DTI measures and are more sensitive to white matter microstructural changes associated with aging and psychiatric disorders (Kodiweera et al. 2016; Chad et al. 2018). A recent longitudinal study utilizing advanced dMRI with NODDI modeling showed altered fiber complexity in patients with a schizophrenia spectrum illness (Kraguljac et al. 2019). Another longitudinal study reported an association between free water and inflammatory burden changes in an animal model of AD (TgF344-AD rats, Fick et al. 2016). Furthermore, a recent study on patients with young-onset Alzheimer’s disease (AD) demonstrated lower ICVF in white matter tracts in both APOE ε4-positive and ε4-negative patients (Slattery et al. 2017). Taken together, NODDI indices may provide novel mechanistic insights regarding healthy aging and early neurodegeneration in white matter microstructure.
On the other hand, in the gray matter, approaches widely used to describe brain structure have characterized differences at the macrostructural level (e.g., brain atrophy) (Fjell et al. 2015; Cantero et al. 2017). While illustrating differences in gross anatomy helps define large-scale brain alterations, it provides little to the underlying neurobiological causes of disease (Nazeri et al. 2020). In contrast, neurite indices can assess underlying neurobiological abnormalities, which may reveal the early disease process and are more likely to provide focal targets for early intervention. Despite the promise of advanced multi-shell dMRI techniques such as NODDI in revealing rich biomarkers for neurological diseases, relatively few studies have utilized this technique (Nazeri et al. 2017; Duchatel et al. 2019; Vogt et al. 2020).
Here, we investigated regional patterns of cortical and white matter microstructure in individuals exhibiting healthy aging as well as in individuals with aMCI, a condition which is typically considered to be prodromal AD in clinical settings. We adopted a gray matter-based spatial statistics (GBSS) approach (Ball et al. 2013) for voxel-wise analysis of NODDI-derived indices within gray matter, using enhanced registration steps and customization to take full advantage of the NODDI model. We further expanded our study to investigate the distribution of neurite properties in cortical surfaces. For the first time, we also examined the relationship between neurite microstructural properties in cortical tissue and in the subjacent white matter tracts in healthy aging to validate the cortical findings. Finally, to demonstrate the sensitivity of the NODDI model in detecting subtle changes in early neurodegeneration, we examined cortical and white matter microstructural alterations in individuals with aMCI and determined the extent to which they predicted cognitive decline.
Materials and Methods
Participants
Forty-five healthy controls (HC) (age range 65–88 years) and 19 age- and education-matched individuals with aMCI (age range 65–85 years) were recruited for this study (Supplementary Table 1). At the time of initial recruitment, all participants were screened for current health status and history of medical and psychiatric conditions via an electronic screening form. Exclusion criteria included left-handedness, presence of suicidality, a formal diagnosis of a significant psychiatric disease, current regular use of psychotropic medications, opiates, or thyroid medications (except for permitted medications including cholinesterase inhibitors and hypertension medications if stable for at least 2 months), claustrophobia, non-MRI-compatible materials, post-traumatic or psychotic disorders, bipolar disorder; any significant neurologic disease including possible and probable dementia, multi-infarct dementia, Parkinson’s or Huntington’s disease, brain tumor, progressive supranuclear palsy, seizure disorder, subdural hematoma, multiple sclerosis, uncontrolled hypertension, history of significant head trauma, current or history of alcohol or substance abuse or dependence within the past 2 years; any significant systemic or unstable medical condition.
Eligible participants underwent a battery of neuropsychological assessments prior to inclusion in the study. The Mini International Neuropsychiatric Interview (M.I.N.I), a structured clinical interview, was used to screen for primary psychiatric conditions. Additionally, potential participants were required to score ≤7 on the geriatric depression scale (GDS), an assessment of depressive symptoms in older adults, score ≥24 on the Mini-Mental State Examination (MMSE), a measure of global cognition, and demonstrate an intact score on the Instrumental Activities of Daily Living (IADL) scale, which assesses functional ability on eight independent activities of daily living. The Clinical Dementia Rating (CDR) was used to categorize participants as either having aMCI (CDR = 0.5) or as HC (CDR = 0).
Measures of memory performance included the Picture Sequence Memory Test (PSMT) (Dikmen et al. 2014) and the Auditory Verbal Learning Test (Rey) (Boake 2000). Processing speed was measured using the Symbol Digit Modalities Test (SDMT) (Weintraub et al. 2013). These measures of memory and processing speed were administered using the National Institutes of Health (NIH) Toolbox (nihtoolbox.org) (for detailed procedures see Supplementary Methods). The study was approved by the Stanford University Institutional Review Board, and all participants provided written informed consent.
MRI Acquisition and Preprocessing
All MRI data were acquired on a 3T GE system (General Electric Healthcare) equipped with a 32-channel head coil (Nova Medical) using a multiband echo-planar imaging (EPI) acquisition scheme (multiband factor of 3, MB = 3) at the Center for Cognitive and Neurobiological Imaging at Stanford University (http://www.cni.stanford.edu/). Multi-shell dMRI data were acquired for all participants, with isotropic 2.0 mm3 spatial resolution in 80 diffusion directions with diffusion gradient strength set to b = 2855 s/mm2 and 30 diffusion directions with diffusion gradient strength b = 710 s/mm2. Each dMRI image also contained nine images without diffusion weighting (b = 0 s/mm2). An additional scan was acquired in the opposite phase encoding direction consisting of 6 diffusion directions (b = 2855 s/mm2) and two non-diffusion-weighted images for EPI distortion correction. Other dMRI parameters are as follows: TR/TE = 2800/78 ms, matrix size = 112 × 112, and 63 axial slices. Preprocessing was implemented in FSL (fsl.fmrib.ox.ac.uk/fsl/fslwiki/) and MRTrix3 (mrtrix.org) and included denoising, geometric EPI distortion (FSL’s topup function), eddy current distortion correction, slice-by-slice motion correction, and outlier detection and bias field correction (ANTs N4BiasField Correction). NODDI coefficients were computed using the NODDI toolbox (https://www.nitrc.org/projects/noddi_toolbox/) in the MATLAB environment. NODDI offers estimates of neurite density (intracellular volume fraction; ICVF) and orientation dispersion index (ODI). Significant advantages of NODDI include the possibility to estimate microstructural properties of white matter and gray matter, allowing for the quantification of neurite morphological changes associated with significant neurological and psychiatric disorders in vivo rather than postmortem (Zhang et al. 2012).
Gray Matter-based Spatial Statistics
Gray matter-based spatial statistics (GBSS) (Ball et al. 2013) was computed using the GBSS pipeline (https://github.com/arash-n/GBSS) (Nazeri et al. 2015). GBSS adapted from tract-based spatial statistics (TBSS) (Smith et al. 2007) was applied to investigate the effects of age on gray matter in a voxel-wise fashion. Gray matter fraction maps were generated by subtracting white matter maps that were estimated from FA maps using the Atropos segmentation tool in ANTs (Avants et al. 2011) and CSF fraction maps (NODDI free water maps). Each tissue map was then multiplied by its respective tissue weighting and added into a pseudo-T1-weighted image (Supplementary Fig. 1). A population-specific T1 template was created using pseudo-T1-weighted images from all participants using the antsMultivariateTemplateConstruction2.sh script in ANTs (Avants et al. 2011). Then, NODDI parameter maps (ICVF and ODI) and gray matter fraction maps in native diffusion space were nonlinearly warped to the population T1 template using the warp fields generated during template construction. Gray matter fraction maps in the T1 template were averaged to create a mean gray matter image that was then skeletonized using the TBSS tool in FSL. ICVF and ODI were then projected onto the gray matter skeleton from local gray matter fraction maxima. Finally, the gray matter skeleton was thresholded to only include with gray matter fraction >0.65.
Figure 2 .
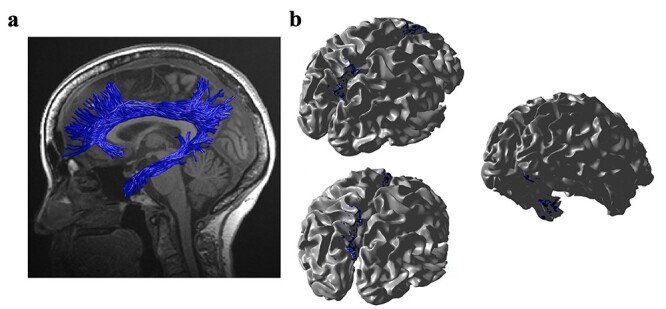
(a) The cingulum fascicle in an individual subject based on probabilistic tractography. (b) The cingulum cingulate and cingulum hippocampus endpoints projected to an individual’s cortical surface.
Surface Mapping
We used FreeSurfer (Version 6.0.0 available at http://surfer.nmr.mgh.harvard.edu) to reconstruct the cortical surfaces of the T1-weighted images using the Human Connectome Project’s multi-modal parcellation (HCPMMP) (Glasser et al. 2016) and more details are provided in Supplementary Methods.
Fiber Tracking and Fiber Tract Segmentation
The fiber orientation distributions (FODs) were generated on the aligned and distortion-corrected dMRI data using multi-shell, and multi-tissue (white matter, gray matter, and CSF) constrain spherical deconvolution with the average tissue response function in MRtrix. Constrained spherical deconvolution was used to estimate the fiber orientation distribution function from the diffusion signal in each voxel. Probabilistic tractography was then used to generate a whole brain tractography, and fiber tract segmentation was performed using the AFQ software package (Yeatman et al. 2012).
Statistical Analysis
All statistical analyses were carried out using scripts written in R (version 3.5.3). The associations between neurite measures (ICVF and ODI) along 19 white matter tracts, in cortical brain regions and age, were modeled by controlling for sex and intracranial brain volume (ICV) using multiple regression. To eliminate the influence of crossing fibers near cortical terminations and to avoid partial volume effects at gray-white matter boundaries, neurite properties along were first averaged over the middle sections (nodes 7–24) of each tract to generate a single mean value of diffusion parameters for each tract. To further investigate whether white matter and cortical neurite properties provide unique microstructural information, we ran ANCOVA models to test the main effect of group by including age, sex and ICV as covariates. Benjamini-Hochberg false discovery rate (FDR) correction was used to account for multiple comparisons across the white matter tracts and cortical ROIs 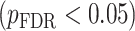 .
.
Results
Microstructural Gray Matter Changes in Relation to Age
Gray Matter-Based Spatial Statistics (GBSS)
GBSS was used to investigate changes in cortical microstructure in healthy older adults using randomize (FSL, 5000 permutations; Nichols and Holmes 2002). In a sample of 45 healthy older adults (32 females) (Supplementary Table 1), we found that age was associated with a widespread decrease in cortical neurite density (ICVF) and neurite orientation dispersion (ODI) (Supplementary Fig. 1). These effects remained stable after inclusion of sex and intracranial brain volume (ICV) in the model (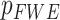 < 0.01).
< 0.01).
Surface-Based Analysis
While voxel-based methods allow highly accurate alignment across subjects, surface-based methods utilizing surface geometry of the cerebral cortex are better suited for multimodal registration (Robinson et al. 2018). Mean cortical ICVF showed high values in early motor and somatosensory areas (Areas 4 and 3a), early visual areas, retroinsular and entorhinal cortex, inferior frontal junction and dorsolateral prefrontal and orbitofrontal cortices (for area description see Supplementary Table 2). A more detailed surface-based region of interest (ROI) analysis revealed a significant widespread decline with advancing age for ICVF (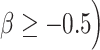 and ODI (
and ODI ( extracted from 360 cortical brain regions (
extracted from 360 cortical brain regions (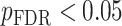 ; Fig. 1). In contrast, we found a significant linear age-related increase in ODI only in the right thalamus and right hippocampus after controlling for sex and intracranial brain volume (ICV) (
; Fig. 1). In contrast, we found a significant linear age-related increase in ODI only in the right thalamus and right hippocampus after controlling for sex and intracranial brain volume (ICV) (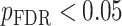 ).
).
Figure 1 .
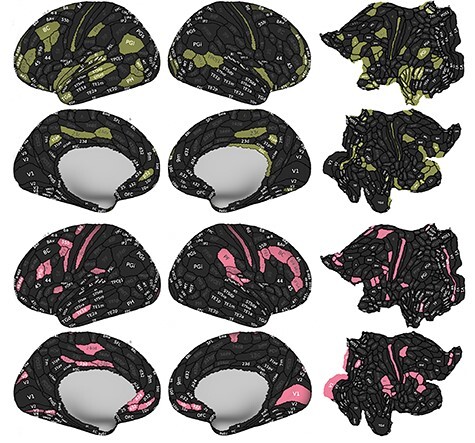
Age association with the ICVF and ODI in gray matter on the left/right lateral and medial, flatten cortical surfaces. ROI results demonstrating significant (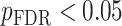 ) ICVF ICVF (green) and ODI (pink) reduction with advancing age through the cortical regions.
) ICVF ICVF (green) and ODI (pink) reduction with advancing age through the cortical regions.
Microstructural White Matter Changes in Relation to Age
We modeled changes in neurite microstructure, ICVF and ODI, for 19 white matter fascicles in the same group of healthy older adults. Lower neurite density (ICVF;  ) was significantly associated with healthy aging across all the tracts (Supplementary Fig. 2), while the white matter neurite complexity (ODI) remained stable in most of the tracts. ICVF most significantly decreased along association tracts, and ODI only reduced in the right and left corticospinal tracts (
) was significantly associated with healthy aging across all the tracts (Supplementary Fig. 2), while the white matter neurite complexity (ODI) remained stable in most of the tracts. ICVF most significantly decreased along association tracts, and ODI only reduced in the right and left corticospinal tracts ( Fig. 4a) with healthy aging.
Fig. 4a) with healthy aging.
Figure 4 .
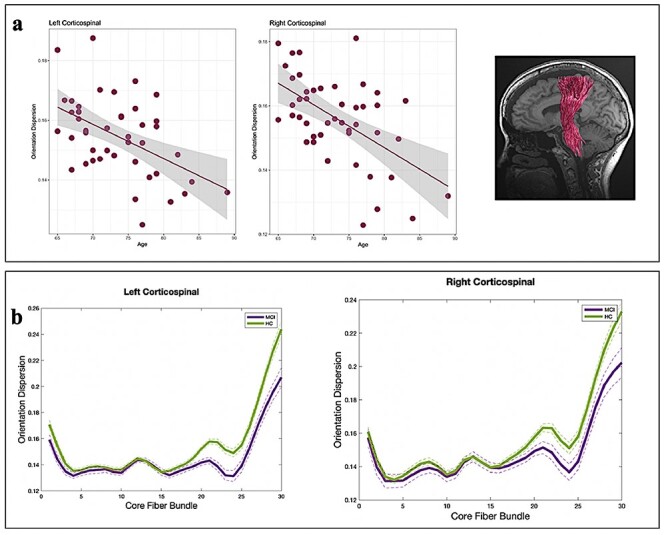
(a) Age associations in neurite orientation dispersion (ODI) in the corticospinal tracts (CST). The solid line denotes the linear regression line, and the width of the grey shade area denotes the 95% confidence interval. (b) ODI measurements in HC and aMCI in the right and left CST. Each solid line represents the group average for ODI, and dotted lines indicate standard error of the mean.
We further investigated the relationship between neurite properties in white matter tracts and cortical terminations associated with those tracts in healthy older adults. We quantified the location and cortical projections of each fascicle with respect to macro anatomical landmarks and observed that mean ICVF in each fascicle was strongly associated (β ≥ 0.6) with ICVF in cortical endpoints ( . For instance, participants with lower ICVF in a given tract tended to have lower ICVF in the majority of cortical endpoints associated with that tract. Figure 2 shows (a) a rendering of the left cingulum cingulate and cingulum hippocampus tracts—both of which are critical pathways underlying memory functioning and have significant implications in aging, aMCI, and AD—and (b) their cortical endpoints (blue color) that are highly correlated with left cingulum cingulate and cingulum hippocampus in ICVF (b). In each hemisphere, the cingulum cingulate tract terminates in the posterior cingulate, anterior cingulate and medial prefrontal cortex, and the cingulum hippocampus fascicle terminates in the medial temporal cortex.
. For instance, participants with lower ICVF in a given tract tended to have lower ICVF in the majority of cortical endpoints associated with that tract. Figure 2 shows (a) a rendering of the left cingulum cingulate and cingulum hippocampus tracts—both of which are critical pathways underlying memory functioning and have significant implications in aging, aMCI, and AD—and (b) their cortical endpoints (blue color) that are highly correlated with left cingulum cingulate and cingulum hippocampus in ICVF (b). In each hemisphere, the cingulum cingulate tract terminates in the posterior cingulate, anterior cingulate and medial prefrontal cortex, and the cingulum hippocampus fascicle terminates in the medial temporal cortex.
Neurite Abnormalities in aMCI
To investigate the alterations of neurite microstructure in aMCI, we first performed ROI analysis that involved 46 bilateral cortical regions known to be implicated in AD and aMCI and extracted ICVF and ODI in these regions relative to age and education matched healthy older adults (HC). We observed a significant decrease in ICVF in the aMCI group in the dorsolateral prefrontal cortex (the area left 9a and right 8BL) and posterior cingulate cortex (the area right 31a) ( Supplementary Fig. 3). In contrast, there were no significant differences in cortical ODI between aMCI and the control group that survived correction for multiple comparisons. Uncorrected P-values indicated that ODI was significantly different within the regions in the medial temporal cortex between groups. The MCI group demonstrated lower ODI in the left hippocampus (H), right entorhinal cortex (EC), and right perirhinal ectorhinal cortex (PeEc) (
Supplementary Fig. 3). In contrast, there were no significant differences in cortical ODI between aMCI and the control group that survived correction for multiple comparisons. Uncorrected P-values indicated that ODI was significantly different within the regions in the medial temporal cortex between groups. The MCI group demonstrated lower ODI in the left hippocampus (H), right entorhinal cortex (EC), and right perirhinal ectorhinal cortex (PeEc) ( ). The aMCI group also showed higher ODI in supplementary motor (6mp, 6ma) and surrounding areas (
). The aMCI group also showed higher ODI in supplementary motor (6mp, 6ma) and surrounding areas (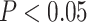 ).
).
Next, we examined differences in ICVF and ODI within four white matter tracts: the left and right cingulum cingulate and cingulum hippocampus between aMCI and HC and observed significant group differences in neurite properties in tracts known to support memory processes. The aMCI group displayed lower ICVF in the right and left cingulum hippocampus compared to HC (Fig. 3a,b). Finally, ODI was significantly increased in aMCI in the right and left corticospinal tracts (CST) in the region where fibers from corpus callosum (CC) pass through the CST (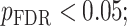 Fig. 4b).
Fig. 4b).
Figure 3 .

(a, b) Neurite density (ICVF) in aMCI and HC in the left and right cingulum hippocampus fascicles. Each solid line represents the group average ICVF, and dashed lines represent the standard error of the mean.
Cognitive Correlates of Neurite Microstructure
To identify whether changes in brain microstructure were accompanied by measurable changes in cognitive performance, we examined the association between white and gray matter microstructure and episodic memory scores, as assessed via Picture Sequence Memory Test (PSMT) and Rey’s Auditory Verbal Learning Test (Rey), as well as processing speed scores measured using the Symbol Digit Modalities Test (SDMT) in aMCI and HC. We only examined the subset of white matter tracts and cortical regions considered crucial for memory circuitry and/or in tracts where aMCI patients demonstrated lower ICVF. SDMT, Rey, and PSMT were positively correlated with ICVF in the right and left cingulum hippocampal fascicles within each group with a stronger association in the HC group (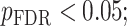 Fig. 5). Similarly, positive associations were found between Rey scores and ICVF in the left dorsolateral prefrontal cortex (area 9a), as well as between SDMT scores and ICVF in the left dorsolateral prefrontal cortex (area 9a) and right posterior cingulate (area 31a) across all participants. Finally, associations between Rey Scores as well as PSMT scores with ICVF in the left dorsolateral prefrontal cortex (area 9a) and right posterior cingulate (area 31a) were significantly different between groups (
Fig. 5). Similarly, positive associations were found between Rey scores and ICVF in the left dorsolateral prefrontal cortex (area 9a), as well as between SDMT scores and ICVF in the left dorsolateral prefrontal cortex (area 9a) and right posterior cingulate (area 31a) across all participants. Finally, associations between Rey Scores as well as PSMT scores with ICVF in the left dorsolateral prefrontal cortex (area 9a) and right posterior cingulate (area 31a) were significantly different between groups (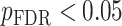 ).
).
Figure 5 .
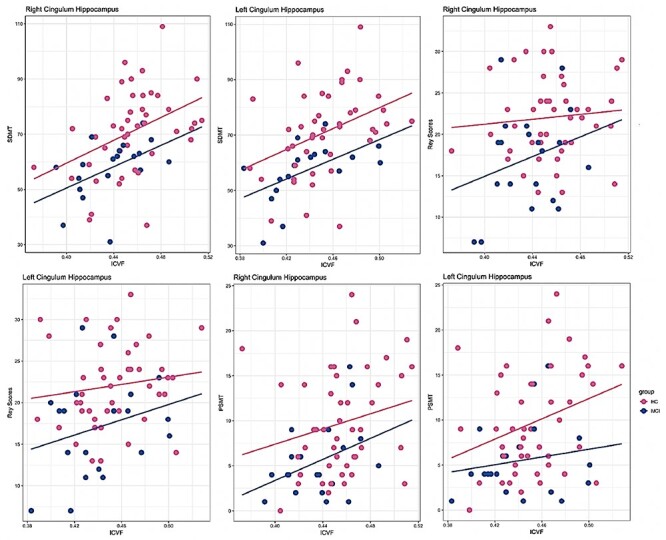
Correlations between mean ICVF in white matter tracts and cognitive performance in aMCI and HC. SDMT: Symbol Digit Modalities Test, PSMT: Picture Sequence Memory Test.
Discussion
Our work identifies microstructural changes in cortical gray and white matter tracts in aMCI and HC using neurite orientation dispersion and density imaging. We found that healthy aging was associated with altered neurite morphology in widespread cortical regions and white matter fascicles. We further demonstrated that aMCI subjects displayed a significant reduction in neurite density in the cortical areas and white matter tracts responsible for episodic memory, among others. These microstructural changes were linked to cognitive performance in healthy aging and aMCI subjects, indicating the clinical utility of neurite imaging in predicting behavioral and cognitive outcomes. Finally, we reported, for the first time, a significant association between neurite density in white matter tracts and in cortical terminations associated with those tracts, further validating our findings.
Current dMRI techniques now allow researchers to study neurite microstructural features in cortical gray matter in healthy aging and neurodegenerative diseases. One recent study observed widespread decreases in ODI with age (ages 21–84 years) but no significant changes in neurite density throughout the cortex (Nazeri et al. 2015). These findings indicate either reduced dendritic complexity or dendritic arborization, and results in this study were particularly pronounced within frontoparietal regions. In contrast, another study (Merluzzi et al. 2016) found decreased neurite density in the cortex, and ODI was not significantly changed with age (ages 45–72 years). Our results are generally in the expected direction, indicating the occurrence of widespread decline in cortical dendritic arborization and neurite density with increasing age. Our results demonstrated some consistency with the previous research, which used much broader or younger age ranges when compared to the current study. Moreover, we also found that ODI increased in the right hippocampus and right thalamus. Intriguingly, the age-related increase in ODI (neurite complexity) observed in the current study has been consistently reported in the hippocampus (Flood et al. 1985; Pyapali and Turner 1996; Nazeri et al. 2015), and the thalamus has connections across the whole cortex, including hippocampal pathways (Behrens et al. 2003; Aggleton et al. 2010). The hippocampus and thalamus are highly myelinated subcortical structures, and ODI increases in the right hippocampus and right thalamus indicate greater disorganization of whiter matter.
Neurite microstructure is a rapidly evolving field in white matter imaging due to clinical feasibility and interpretability. This technique provides biophysical models that are useful in the investigation of both healthy and pathological aging. We demonstrated a strong negative association between healthy aging and neurite density across all white matter tracts and ODI in the right and left CST tracts, and our results are in line with the previous findings (Cox et al. 2016; Merluzzi et al. 2016). However, the negative age association with ODI is inconsistent with previous evidence, which found a positive association between age and ODI within CST tracts (Kodiweera et al. 2016). The apparent difference between studies might be attributed to the following factors. The previous study examined a younger cohort than the current sample in this study, whereas a negative association was found (Merluzzi et al. 2016) when studying older participants. Also, we found negative age associations only in the CST tracts. These tracts are known to have many crossing fibers that may contribute to the discrepancy (Wedeen et al. 2008). Thus, comparability should be considered in light of the specific tract location and age considered.
Neurite health in older age is thought to impact the likelihood of developing neurodegenerative disorders such as AD (Colgan et al. 2016; Vogt et al. 2020). Investigations of neurite microstructure commonly focused on the white matter tracts in MCI and AD patients. One recent study identified decreased neurite density in white matter tracts in MCI and AD while there were no significant changes in fractional anisotropy in MCI patients (Fu et al. 2020). Also, neurite density was highly correlated with tau pathology in a mouse model of AD (Colgan et al. 2016). We also found significantly reduced neurite density in the left and right cingulum hippocampus fascicles in aMCI compared with HC. However, only a few studies have assessed these neurite parameters in MCI and AD, reporting lower neurite density in the temporal and parietal cortical regions in MCI as well as across temporal, parietal, and frontal cortical regions in patients with AD (Vogt et al. 2020). Similarly, we found that neurite density was decreased in the regions located within prefrontal and temporal areas and in the posterior cingulate cortex, consistent with the spread of the AD pathology through the association cortex. Neurite density has a high degree of correlation with myelin mapping, and this biophysical model is based on an assumption regarding cellular structure outperformed the tensor model (Jespersen et al. 2010). Thus, the field is moving ever closer to accurate estimates of a direct measure of neural morphometry in white and gray matter across neurodegenerative disorders, particularly having high implications for the early detection of AD. However, much more work is needed to establish the accuracy and robustness of these measures both in white and gray matter.
Interestingly, despite having lower ICVF in all fascicles and higher ODI across most tracts, the aMCI group had significantly lower ODI in the left and right CST tracts—the white matter motor pathways compared to healthy controls. At first glance, the difference might appear counterintuitive because increased ODI in white matter represents an increasing misalignment of axons due to the accumulation of both intra- and extracellular detritus. However, the aMCI group displayed decreased ODI in the region that contains interpenetration of association, projection, and commissural fibers. Previous studies have reported that reduced ODI is compatible with reduced arborization or connectivity within the highly dispersed axons and dendritic regions (Fukutomi et al. 2018). It should be noted that biophysical heterogeneity within motor regions contributes to difficulties in detecting consistent alterations using current imaging techniques. Thus, these findings indicate that ODI can reveal a new aspect of the cellular pathology of motor pathways and disclose early microstructural changes in aMCI.
Characterizing the cortical connections in white matter fascicles and their tissue microstructures in the living human brain is crucial for cognitive neuroscience. For example, the cingulum fascicle is known to have a complex structure and contributes to various functions because of its extensive structural connections with many cortical brain regions (Bubb et al. 2018). However, the signal carried by the cingulum fascicles critically affects cortical endpoints remains unclear and can be resolved via the integration of multiple imaging modalities. In this study, we found that neurite density in the cingulum cingulate and cingulum hippocampus tracts correlated with cortical neurite density in the regions located in each fascicle’s endpoints using advanced dMRI. Our findings demonstrate how microstructural changes in white matter fascicles could contribute to fiber density changes in cortical tract terminations. They further validate our cortical microstructural measurements considering the complexity of neurite measurements in the cortex. Thus, neurite density could consolidate our understanding of how white matter biophysical parameters interact with their cortical substrates. Next, we tested whether episodic memory scores were related to microstructural changes in the cortex and white matter. We predicted a priori that episodic memory scores would be selectively associated with specific regional brain microstructure, consistent with prior work (Nazeri et al. 2015). We found that neurite density and episodic memory scores were positively correlated, both in the cortex and white matter tracts, in healthy older adults as well as in aMCI patients. In summary, this study uncovers the critical role of neurite density to illuminate biological mechanisms linking white matter and cortical changes to behavior in healthy aging and aMCI.
To conclude, we found that healthy aging was associated with microstructural changes in neurite morphology, which were present focally in aMCI patients both in the cortex and white matter. We also demonstrated the utility of neurite density metrics for detecting and quantifying cortical and white matter changes associated with aMCI and how these biophysical changes were linked to episodic memory. Crucially, the present study also highlights that white matter neurite microstructural changes are highly predictive of cortical neurite morphology characteristics in healthy aging and may be used to monitor disease progression in future studies.
Supplementary Material
Supplementary material can be found at Cerebral Cortex online.
Author Contributions
E.G. contributed to M.R.I. data collection, carried out image processing and statistical analysis, and drafted the manuscript. H.F. and L.D. contributed to recruitment, study coordination, behavioral data collection and analysis, M.R.I. data collection, and drafting the manuscript. J.L.B. contributed to drafting the manuscript. S.M.H.H. conceived of the study, participated in its design and coordination, and helped with drafting the manuscript. All authors read and approved the final manuscript.
Funding
Career Development Award from the National Institute on Aging (NIA) (K25AG050759 to S.M.H.H); Stanford Maternal and Child Health Research Institute (MCHRI) (to E.G.); National Institute on Aging (NIA) (R21AG064263 to S.M.H.H.); National Institute of Mental Health (NIMH) (R61MH119289, R21MH123873 to S.M.H.H.).
Notes
We thank the participants for their involvement in the study as well as the researchers involved in coordinating data collection for this project.
Supplementary Material
Contributor Information
Elveda Gozdas, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
Hannah Fingerhut, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
Lauren Dacorro, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
Jennifer L Bruno, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
S M Hadi Hosseini, Department of Psychiatry and Behavioral Sciences, Stanford University School of Medicine, Stanford, CA 94304, USA.
References
- Aggleton JP, O’Mara SM, Vann SD, Wright NF, Tsanov M, Erichsen JT. 2010. Hippocampal–anterior thalamic pathways for memory: uncovering a network of direct and indirect actions. Eur J Neurosci. 31:2292–2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Tustison NJ, Wu J, Cook PA, Gee JC. 2011. An open source multivariate framework for n-tissue segmentation with evaluation on public data. Neuroinformatics. 9:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ball G, Srinivasan L, Aljabar P, Counsell SJ, Durighel G, Hajnal JV, Rutherford MA, Edwards AD. 2013. Development of cortical microstructure in the preterm human brain. Proc Natl Acad Sci. 110:9541–9546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TE, Johansen-Berg H, Woolrich M, Smith S, Wheeler-Kingshott C, Boulby P, Barker G, Sillery E, Sheehan K, Ciccarelli O. 2003. Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci. 6:750–757. [DOI] [PubMed] [Google Scholar]
- Boake C. 2000. Edouard Claparede and the auditory verbal learning test. J Clin Exp Neuropsychol. 22:286–292. [DOI] [PubMed] [Google Scholar]
- Bubb EJ, Metzler-Baddeley C, Aggleton JP. 2018. The cingulum bundle: anatomy, function, and dysfunction. Neurosci Biobehav Rev. 92:104–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantero JL, Zaborszky L, Atienza M. 2017. Volume loss of the nucleus basalis of meynert is associated with atrophy of innervated regions in mild cognitive impairment. Cereb Cortex. 27:3881–3889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chad JA, Pasternak O, Salat DH, Chen JJ. 2018. Re-examining age-related differences in white matter microstructure with free-water corrected diffusion tensor imaging. Neurobiol Aging. 71:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colgan N, Siow B, O'Callaghan JM, Harrison IF, Wells JA, Holmes HE, Ismail O, Richardson S, Alexander DC, Collins EC. 2016. Application of neurite orientation dispersion and density imaging (NODDI) to a tau pathology model of Alzheimer's disease. NeuroImage. 125:739–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox SR, Ritchie SJ, Tucker-Drob EM, Liewald DC, Hagenaars SP, Davies G, Wardlaw JM, Gale CR, Bastin ME, Deary IJ. 2016. Ageing and brain white matter structure in 3,513 UK Biobank participants. Nat Commun. 7:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickstein DL, Weaver CM, Luebke JI, Hof PR. 2013. Dendritic spine changes associated with normal aging. Neuroscience. 251:21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikmen SS, Bauer PJ, Weintraub S, Mungas D, Slotkin J, Beaumont JL, Gershon R, Temkin NR, Heaton RK. 2014. Measuring episodic memory across the lifespan: NIH toolbox picture sequence memory test. JINS. 20:611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duchatel RJ, Weickert CS, Tooney PA. 2019. White matter neuron biology and neuropathology in schizophrenia. NPJ Schizophr. 5:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fick RH, Daianu M, Pizzolato M, Wassermann D, Jacobs RE, Thompson PM, Town T, Deriche R. 2016. Comparison of biomarkers in transgenic alzheimer rats using multi-shell diffusion MRI. In: International Conference on Medical Image Computing and Computer-Assisted Intervention. Cambridge: Springer, pp. 187–199. [Google Scholar]
- Fjell AM, Grydeland H, Krogsrud SK, Amlien I, Rohani DA, Ferschmann L, Storsve AB, Tamnes CK, Sala-Llonch R, Due-Tønnessen P. 2015. Development and aging of cortical thickness correspond to genetic organization patterns. Proc Natl Acad Sci. 112:15462–15467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flood DG, Buell SJ, Defiore CH, Horwitz GJ, Coleman PD. 1985. Age-related dendritic growth in dentate gyrus of human brain is followed by regression in the ‘oldest old’. Brain Res. 345:366–368. [DOI] [PubMed] [Google Scholar]
- Fukutomi H, Glasser MF, Zhang H, Autio JA, Coalson TS, Okada T, Togashi K, Van Essen DC, Hayashi T. 2018. Neurite imaging reveals microstructural variations in human cerebral cortical gray matter. NeuroImage. 182:488–499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu X, Shrestha S, Sun M, Wu Q, Luo Y, Zhang X, Yin J, Ni H. 2020. Microstructural white matter alterations in mild cognitive impairment and Alzheimer’s disease. Clin Neuroradiol.30:569–579. [DOI] [PubMed] [Google Scholar]
- Glasser MF, Coalson TS, Robinson EC, Hacker CD, Harwell J, Yacoub E, Ugurbil K, Andersson J, Beckmann CF, Jenkinson M. 2016. A multi-modal parcellation of human cerebral cortex. Nature. 536:171–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jespersen SN, Bjarkam CR, Nyengaard JR, Chakravarty MM, Hansen B, Vosegaard T, Østergaard L, Yablonskiy D, Nielsen NC, Vestergaard-Poulsen P. 2010. Neurite density from magnetic resonance diffusion measurements at ultrahigh field: comparison with light microscopy and electron microscopy. NeuroImage. 49:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kodiweera C, Alexander AL, Harezlak J, McAllister TW, Wu Y-C. 2016. Age effects and sex differences in human brain white matter of young to middle-aged adults: a DTI, NODDI, and q-space study. NeuroImage. 128:180–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraguljac NV, Anthony T, Monroe WS, Skidmore FM, Morgan CJ, White DM, Patel N, Lahti AC. 2019. A longitudinal neurite and free water imaging study in patients with a schizophrenia spectrum disorder. Neuropsychopharmacology. 44:1932–1939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merluzzi AP, Dean DC III, Adluru N, Suryawanshi GS, Okonkwo OC, Oh JM, Hermann BP, Sager MA, Asthana S, Zhang H. 2016. Age-dependent differences in brain tissue microstructure assessed with neurite orientation dispersion and density imaging. Neurobiol Aging. 43:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Chakravarty MM, Rotenberg DJ, Rajji TK, Rathi Y, Michailovich OV, Voineskos AN. 2015. Functional consequences of neurite orientation dispersion and density in humans across the adult lifespan. J Neurosci. 35:1753–1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazeri A, Mulsant BH, Rajji TK, Levesque ML, Pipitone J, Stefanik L, Shahab S, Roostaei T, Wheeler AL, Chavez S. 2017. Gray matter neuritic microstructure deficits in schizophrenia and bipolar disorder. Biol Psychiatry. 82:726–736. [DOI] [PubMed] [Google Scholar]
- Nazeri A, Schifani C, Anderson JA, Ameis SH, Voineskos AN. 2020. In vivo imaging of gray matter microstructure in major psychiatric disorders: opportunities for clinical translation. Biol Psychiat Cogn Neurosci Neuroimaging. 5:855–864. [DOI] [PubMed] [Google Scholar]
- Nichols TE, Holmes AP. 2002. Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp. 15:1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyapali GK, Turner DA. 1996. Increased dendritic extent in hippocampal CA1 neurons from aged F344 rats. Neurobiol Aging. 17:601–611. [DOI] [PubMed] [Google Scholar]
- Rathi Y, Pasternak O, Savadjiev P, Michailovich O, Bouix S, Kubicki M, Westin CF, Makris N, Shenton ME. 2014. Gray matter alterations in early aging: a diffusion magnetic resonance imaging study. Hum Brain Mapp. 35:3841–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EC, Garcia K, Glasser MF, Chen Z, Coalson TS, Makropoulos A, Bozek J, Wright R, Schuh A, Webster M. 2018. Multimodal surface matching with higher-order smoothness constraints. NeuroImage. 167:453–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slattery CF, Zhang J, Paterson RW, Foulkes AJ, Carton A, Macpherson K, Mancini L, Thomas DL, Modat M, Toussaint N. 2017. ApoE influences regional white-matter axonal density loss in Alzheimer's disease. Neurobiol Aging. 57:8–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Johansen-Berg H, Jenkinson M, Rueckert D, Nichols TE, Miller KL, Robson MD, Jones DK, Klein JC, Bartsch AJ. 2007. Acquisition and voxelwise analysis of multi-subject diffusion data with tract-based spatial statistics. Nat Protoc. 2:499. [DOI] [PubMed] [Google Scholar]
- Vogt NM, Hunt JF, Adluru N, Dean DC III, Johnson SC, Asthana S, J-PJ Y, Alexander AL, Bendlin BB. 2020. Cortical microstructural alterations in mild cognitive impairment and Alzheimer’s disease dementia. Cereb Cortex. 30:2948–2960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedeen VJ, Wang R, Schmahmann JD, Benner T, Tseng W-YI, Dai G, Pandya D, Hagmann P, D'Arceuil H, de Crespigny AJ. 2008. Diffusion spectrum magnetic resonance imaging (DSI) tractography of crossing fibers. NeuroImage. 41:1267–1277. [DOI] [PubMed] [Google Scholar]
- Weintraub S, Dikmen SS, Heaton RK, Tulsky DS, Zelazo PD, Bauer PJ, Carlozzi NE, Slotkin J, Blitz D, Wallner-Allen K. 2013. Cognition assessment using the NIH toolbox. Neurology. 80:S54–S64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeatman JD, Dougherty RF, Myall NJ, Wandell BA, Feldman HM. 2012. Tract profiles of white matter properties: automating fiber-tract quantification. PLoS One. 7:e49790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Schneider T, Wheeler-Kingshott CA, Alexander DC. 2012. NODDI: practical in vivo neurite orientation dispersion and density imaging of the human brain. NeuroImage. 61:1000–1016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


