FIGURE 4.
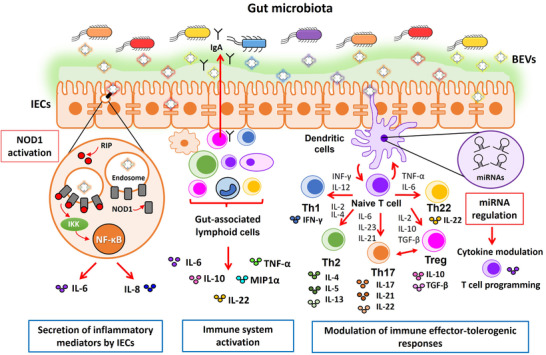
Schematic picture summarizing the immunomodulatory effects elicited by microbiota‐derived BEVs in the gut. The drawing shows the intestinal epithelium covered with the mucin layer that prevents access of luminal microbes while allowing passage of BEVs. Immune cells (lymphocytes, macrophages and dendritic cells) in the lamina propria are shown below the epithelial monolayer. Microbiota derived BEVs exert immune modulation by two main mechanisms. (i) Undirect activation of immune cells through the intestinal epithelium (left scheme). Internalized EVs by intestinal epithelial cells (IECs) activate the cytosolic receptor NOD1 that triggers secretion of immune effectors, which in turn stimulate gut‐associated lymphoid cells to produce a wide range of cytokines. Activation of the NOD1 signalling pathway by microbiota BEVs is shown encircled in more detail. BEVs are internalized through clathrin‐mediated endocytosis and recruit NOD1 (grey cylinders) to early endosomes. Activated NOD1 interacts with the specific kinase RIP2 (red circles), which leads to NF‐kB activation and the subsequent upregulation of host genes involved in the inflammatory response (IL‐6, IL‐8). (ii) Direct activation of gut resident immune cells by microbiota BEVs that leads to secretion of immune mediators and secretory IgA (middle scheme). In addition to direct interaction with microbiota BEVs that reach the gut‐associated lymphoid tissue via transcytosis across M cells, dendritic cells (DCs) also interact with luminal BEVs by extending pseudopodia across the epithelial cell layer (right scheme). Studies with several gut microbiota species revealed that BEVs activate DCs in a strain‐specific manner. Differential regulation of miRNAs in DCs is one of the regulatory mechanisms involved in the specific immunomodulatory effects of BEVs isolated from probiotic and commensal E. coli is (highlighted in the adjacent violet circle). In conclusion, DCs integrate incoming signals delivered by microbiota BEVs and set up specific programs that promote differentiation of naïve T cells into effector T cells (Th1, Th2, Th17, Th22) or regulatory T cells (Treg), thus allowing coordination of suitable T cell responses
