FIGURE 3.
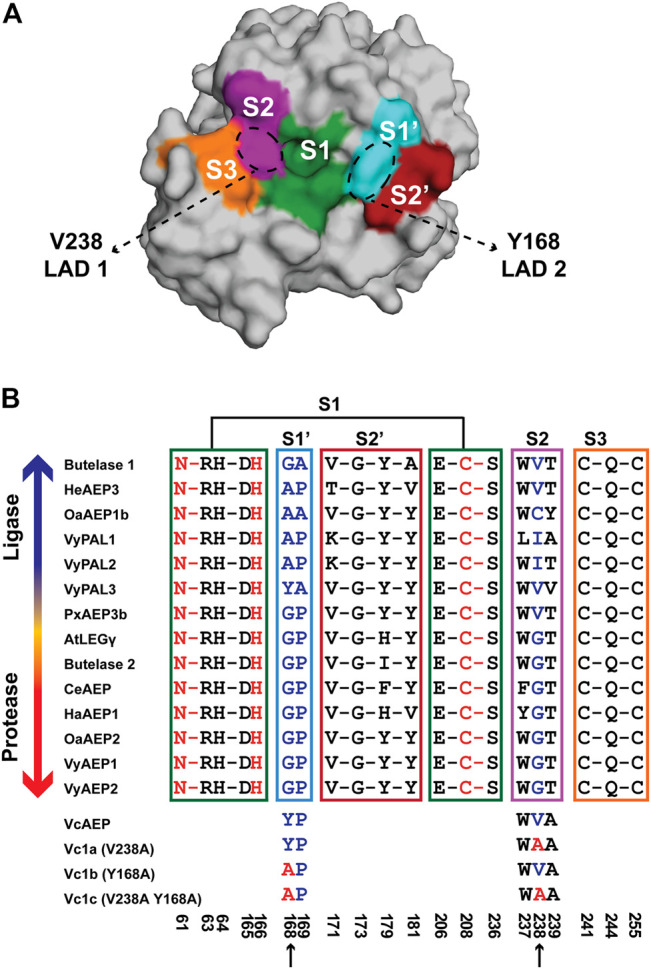
Engineering a ligase from a dual-functional AEP, VcAEP. (A) Proposed substrate binding pockets (S3, S2, S1, S1′, S2′) based on the crystal structure of the VcAEP proform (pdb: 5ZBI). The S3 pocket (orange) comprises residues C241, Q244, and C255, S2 (purple) W237, V238, and A239, S1 (green) N61, R63, H64, D165, H166, E206, C208, and S236, S1′ (cyan) Y168 and P169, S2′ (red) V171, G173, Y179, and Y181. (B) Sequence alignment of substrate-binding pockets of known AEPs for which protease activity dominates (e.g., CeAEP, butelase-2), and PALs having dominant ligase activity (e.g., butelase-1, VyPAL2). The blue arrow indicates increasing ligase activity and the red arrow indicates increasing protease activity. The three engineered VcAEP mutants having mutations at S2 (V238 using VcAEP numbering) and S1′ (Y168) sites are Vc1a (V238A), Vc1b (Y168A), and Vc1c (V238A Y168A). Color codes are the same as for (A) and (B) with S3 in orange, S2 in purple, S1 in green, S1′ in cyan, and S2 in red. The catalytic triad N61, H166, and C208 in the oxyanion hole corresponding to S1 is colored red. Key residues in the S1′ and S2 pockets for steering AEP or PAL activities (panel B) are respectively colored cyan for Y168P169, and purple for V238. Residues comprising the catalytic S1, S1′, S2′, S2 and S3 pockets are framed by a green, cyan, red, purple and orange box, respectively. Three VcAEP mutants with increasing ligase activity were engineered that target S2 and S1′ pockets (indicated by black arrows).
