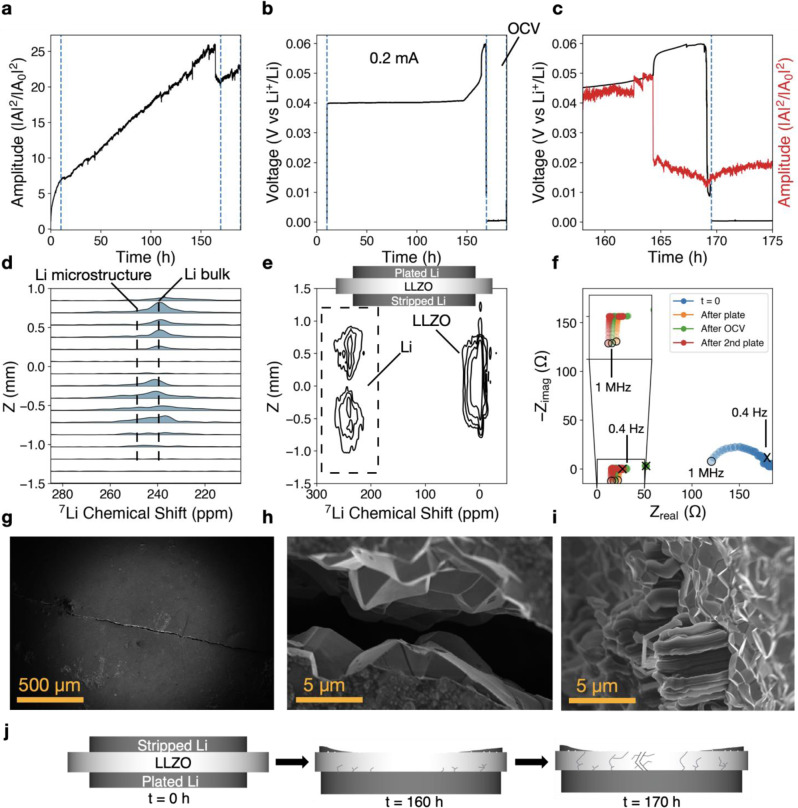
Fig. 4. Lithium metal–LLZO interface at 13 MPa.
Polarization test of Li (s)–LLZO interface at 13 MPa stack pressure conducted in a Li/LLZO/Li cell. After a few hours of initial mechanical equilibration, 0.2 mA/cm2 is applied galvanostatically until the voltage cutoff of 5 V, followed by a 25 h OCV rest step, a second polarization step in the same direction, and a second OCV rest step. a Normalized acoustic amplitude. b Voltage showing increase in overpotential until an electrochemical short at ~60 mV vs Li+/Li (t ~ 169 h) during the galvanostatic current step of 0.2 mA/cm2. Vertical guidelines (blue dotted lines) are shown as a visual aid to delineate start and stop of galvanostatic current. c Zoomed in region of electrochemical shorting event at around t = 169 h which is accompanied by a sudden attenuation of acoustic amplitude. d 1D slices of ex situ 7Li CSI highlighting the lithium metal peak between 200 and 280 ppm, as indicated by the dashed region in e. e Ex situ 7Li CSI contour plot showing formation of lithium microstructures at the Li (s)–LLZO interface for both electrodes, and the presence of bulk lithium metal (240 ppm) in the electrolyte. f Nyquist plot from potentiostatic electrochemical impedance spectroscopy conducted during initial OCV, after galvanostatic current to the voltage cutoff, after the 25 h OCV, and after the second plating step to the voltage cutoff. The open circle indicates the high-frequency start point (1 MHz), and the cross indicates the low-frequency end point (0.4 Hz). g SEM image of center of LLZO on the stripped lithium side (in this case, most of the existing lithium foil was plated through the electrolyte resulting in an almost-bare LLZO surface), showing a large crack. h High-resolution SEM image of crack which reveals the polycrystalline grains of LLZO. i High-resolution SEM image showing the presence of lithium protrusions from polycrystalline grain boundaries along the exposed crack, with protrusions oriented parallel to the electrode (instead of protruding through the electrolyte). j Simplified cartoon illustration of interface behavior implied by the experimental data; not drawn to scale.