Abstract
Background
Ginsenoside Rg3, isolated from Panax ginseng, has anti-inflammatory and anti-tumor activities. It is known to reduce inflammation in acute lung injury in mice, and to reduce the expression of inflammatory cytokines and COX-2 in human asthmatic airway epithelium. In this study, we attempted to determine whether ginsenoside Rg3 inhibits airway inflammation, oxidative stress, and airway hyperresponsiveness (AHR) in the lungs of asthmatic mice. We also investigated its effects on oxidative stress and the inflammatory response in tracheal epithelial cells.
Methods
Asthma symptoms were induced in female BALB/c mice sensitized with ovalbumin (OVA). Mice were divided into five groups: normal controls, OVA-induced asthmatic controls, and asthmatic mice treated with ginsenoside Rg3 or prednisolone by intraperitoneal injection. Inflammatory BEAS-2B cells (human tracheal epithelial cells) treated with ginsenoside Rg3 to investigate its effects on inflammatory cytokines and oxidative responses.
Results
Ginsenoside Rg3 treatment significantly reduced eosinophil infiltration, oxidative responses, airway inflammation, and AHR in the lungs of asthmatic mice. Ginsenoside Rg3 reduced Th2 cytokine and chemokine levels in bronchoalveolar lavage fluids and lung. Inflammatory BEAS-2B cells treated with ginsenoside Rg3 reduced the eotaxin and pro-inflammatory cytokine expressions, and monocyte adherence to BEAS-2B cells was significantly reduced as a result of decreased ICAM-1 expression. Furthermore, ginsenoside Rg3 reduced the expression of reactive oxygen species in inflammatory BEAS-2B cells.
Conclusion
Ginsenoside Rg3 is a potential immunomodulator that can ameliorate pathological features of asthma by decreasing oxidative stress and inflammation
Keywords: Airway hyperresponsiveness, Asthma, Eosinophil, Ginsenoside Rg3, Oxidative stress
Graphical abstract
Highlights
-
•
Ginsenoside Rg3 reduced eosinophil infiltration, and airway hyperresponsiveness in the lungs of asthmatic mice.
-
•
Ginsenoside Rg3 inhibited oxidative responses in the lungs.
-
•
Ginsenoside Rg3 reduced the levels of Th2 cytokines in BALF and lung.
-
•
Ginsenoside Rg3 inhibited monocyte cell adherence to tracheal epithelial cells.
-
•
Ginsenoside Rg3 reduced the levels of pro-inflammatory cytokines in tracheal epithelial cells.
1. Introduction
Asthma is a chronic allergic and inflammatory disease of the airways. Exposure to allergens or microbes can induce asthma attacks [1]. The clinical symptoms of asthma are complex, with chest tightness, cough, shortness of breath, and dyspnea being the main symptoms during an acute asthma attack [2]. Allergens stimulate the immune system to exacerbate airway narrowing by smooth muscle contraction during asthma attacks, and airway epithelial cells also secrete more mucus, which obstructs the airways [3]. Lung infiltration by a large number of inflammatory immune cells (especially eosinophils) causes more severe respiratory inflammation and allergic symptoms, and also increases airway hyperresponsiveness (AHR), causing shortness of breath and dyspnea [2]. These pathological symptoms of asthma are related to Th2 cell activation, which releases massive amounts of IL-4, IL-13, and IL-5 [4]. These cytokines induce excessive secretion of IgE and trigger the activation of immune cells. They also contribute to excessive secretion of mucus, AHR, and airway remodeling [5].
Airway epithelial cells secrete mucus that traps inhaled microorganisms and allergens, and these cells also regulate the allergic and inflammatory reactions caused by exposure to these environmental substances [6]. These cells release more inflammatory cytokines, which exacerbate respiratory system inflammation, and chemokines, which attract immune cells to infiltrate the lungs [7]. Inflamed tracheal epithelial cells can activate oxidase expression to induce reactive oxygen species (ROS) and free radical production, causing oxidative stress and consequent lung damage [7].
Panax ginseng is distributed throughout China and Korea, and its root is one of the most widely used herbs for Qi-invigorating therapy [8]. Both Korean Red Ginseng and white ginseng have been demonstrated to improve asthma in mice, and Korean Red Ginseng improves asthma better than Korean white ginseng [9]. Ginsenosides are the main biologically active compounds of ginseng. Known ginsenosides include Rb1, Rb3, Rg1, Rc, Rh2, Rg2, and Rg3 [10,11]. Ginsenoside Rg3 could reduce acute lung injury by activating PI3K/AKT/mTOR and suppressing NF-κB signaling pathways [12,13]. Ginsenoside Rg3 also reduced inflammatory mediators in IL-1β–activated A549 lung epithelial cells by blocking NF-κB signaling pathways [14]. Furthermore, Rb1 and Rh2 improved AHR and airway inflammation in asthmatic mice by modulating Th2-cell activity and NF-κB signaling [15,16]. However, the effects of ginsenoside Rg3 on AHR, inflammation, and oxidative stress are unclear. In the current study, we would evaluate the ability of ginsenoside Rg3 to ameliorate asthma symptoms, and investigated the effects of ginsenoside Rg3 on immune function, oxidative stress, and inflammation in asthmatic mice.
2. Material and methods
2.1. Animals
Female BALB/c mice (20–25 g of body weight), 6 weeks of age, were purchased from the National Laboratory Animal Center (Taiwan). Mice were kept in an air-conditioned animal room with a 12-hour light/dark cycle and allowed to consume water and standard chow diet ad libitum. Animal experiments were conducted in accordance with the Animal Care and Protection Committee of Chang Gung University of Science and Technology (Approval number: 2014-007).
2.2. Drug treatment and sensitization
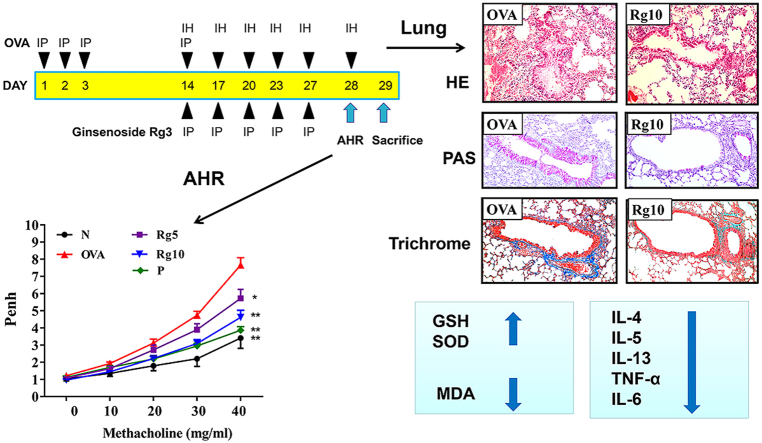
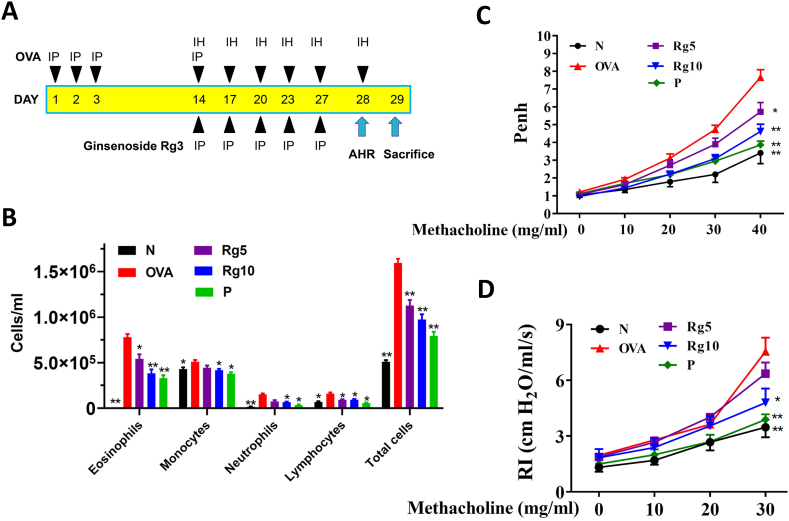
20(S)-ginsenoside Rg3 (≥98% purity) was purchased from Sigma-Aldrich Co. (St. Louis, MO, USA), and was dissolved in DMSO solution. The experimental protocol for sensitized asthma mouse model is shown in Fig. 1A. Recent studies have demonstrated that 10mg/kg ginsenoside Rg3 could attenuates LPS-induced acute lung injury in mice [17]. Hence, subsequent experiments used ginsenoside Rg3 at 5 and 10mg/kg for all animal experiments. Briefly, the mice were sensitized using ovalbumin (OVA; Sigma) sensitizing solution (50 μg OVA in 200 μL normal saline containing 0.8 mg (AlOH3) adjuvant), administered by intraperitoneal injection on days 1–3 and day 14. Next, mice inhaled 2% atomized OVA to challenge lung allergy on days 14, 17, 20, 23, and day 27. Mice gave with prednisolone, ginsenoside Rg3, or DMSO by intraperitoneal injection 1 h before methacholine inhalation or OVA challenge. Experimental mice were tested for AHR by methacholine inhalation on day 28. On day 29, mice were sacrificed to investigate oxidative response, inflammatory, immunomodulatory, and asthma pathology symptoms. The mice divided into five groups (n = 10 in each group): (A) normal control (N group), mice treated with 50 μL DMSO by intraperitoneal injection; (B) OVA-sensitized control (OVA group), mice sensitized with OVA and treated with 50 μL DMSO by intraperitoneal injection; (C) the prednisolone control (P group), OVA-sensitized mice treated with 5 mg/kg prednisolone (dissolved in DMSO) by intraperitoneal injection; and (D) the OVA-sensitized mice treated with 5 mg/kg or 10 mg/kg ginsenoside Rg3 (dissolved in DMSO) by intraperitoneal injection (Rg5 and Rg10 groups, respectively).
Fig. 1.
Effect of ginsenoside Rg3 on AHR and cell counts in BALF from asthmatic mice. (A) Experimental procedures for asthmatic mouse studies. (B) Inflammatory cells in BALF were counted. (C) Mice inhaled increasing doses of methacholine, and AHR was assessed and is shown as Penh values. (D) AHR was measured as a percentage from the baseline level of lung resistance (RI). Three independent experiments were analyzed, and all data are presented as the mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01 compared with the OVA control group. Mice were divided into normal (N), OVA-sensitized mice (OVA), 5 mg/kg prednisolone control (P), 5 mg/kg ginsenoside Rg3 (Rg5) and 10 mg/kg ginsenoside Rg3 (Rg10) groups. IP, intraperitoneal injection; IH, inhalation.
2.3. AHR assay
Mice tested for AHR evaluated using airway flow and function, as described previously [18]. Briefly, mice were exposed to inhale an increasing dose of aerosolized (0 to 40 mg/mL) methacholine for 3 min. Next, mice were placed in a closed chamber, where the enhanced pause (Penh) signal data were measured in order to determine AHR values, using the whole-body plethysmograph system (Buxco Electronics, Troy, NY, USA). Furthermore, the mice were anesthetized and intubated to evaluate their lung function by detecting respiratory resistance by use of a low-frequency forced oscillation technique (Buxco Electronics) [19].
2.4. Malondialdehyde (MDA)
MDA was detected using the lipid peroxidation assay kit (Sigma) as described previously [20]. Mice lungs were removed and homogenized (FastPrep-24, MP Biomedicals, Santa Ana, CA, USA). The tissue solution was treated with perchloric acid and centrifuged to collect the supernatant. Finally, the level of MDA was assayed by a multi-mode microplate reader (SpectraMax i3X, Molecular Devices, San Jose, CA, USA).
2.5. Glutathione (GSH) and superoxide dismutase (SOD) assay
The levels of glutathione in lung tissues were detected using a glutathione assay kit (Sigma), and SOD activity was assayed using a SOD determination kit (Sigma) according to the manufacturer’s instructions as described previously [21]. The GSH and SOD levels were detected using a microplate reader (Multiskan FC, Thermo, Waltham, MA, USA).
2.6. Serum collection and splenocyte culture
Mice were anesthetized with 4% isoflurane, and blood was harvested from the orbital vascular plexus. The sample centrifuged to collect the serum in order to detect OVA-specific antibodies, as described previously [22]. The spleens were removed, and splenocytes (5 × 106 cells/mL) seeded on 24-well culture plates containing 100 μg/mL OVA and grown for 5 days. The supernatant was assayed for cytokines as described previously [23].
2.7. Bronchoalveolar lavage fluid (BALF)
Mice were sacrificed, and BALF collected as described previously [24]. Briefly, using an indwelling needle intubating the trachea, lungs were washed with 1 mL sterile normal saline. The BALF collected and chemokines and cytokines were assayed by use of an ELISA. Next, BALF centrifuged using cytospin centrifugation (Cytospin 4, Thermo), and BALF cells stained with Giemsa stain (Sigma) for counting and to record cell morphology.
2.8. Histopathological assessment
Lung was fixed with formalin, and embedded in paraffin. Lung sections stained with periodic acid-Schiff solution (PAS; Sigma) to investigate tracheal goblet cell hyperplasia, as described previously [21]. The lung tissue sections stained with hematoxylin and eosin (HE) to observe eosinophil infiltration. The pathology scores of eosinophil infiltration were evaluated using a five-point grading system described previously [25]. Lung tissue stained with Masson’s trichrome solution (Sigma) to observed collagen expression, as described previously [19].
2.9. Immunohistochemical staining
Lungs embedded in paraffin and sectioned, and the sections treated with COX-2 antibody (Abcam, Cambridge, UK) overnight. The slides were then treated with secondary antibodies and incubated with DAB substrate to detect COX-2 expression, as described previously [26].
2.10. ELISA assay
Serum OVA-specific antibodies were detected by use of an ELISA kit (BD Biosciences, Franklin Lakes, NJ, USA) [24]. Serum diluted 5-fold to evaluate OVA-IgE expressions as the OD450 values. The units of OVA-IgG2a and OVA-IgG1were defined using OD450 values from standard curves of pooled serum from OVA-sensitized mice. The levels of chemokines and cytokines in cell culture media and BALF were detected using the specific ELISA kit (R&D Systems, Minneapolis, MN, USA) [27].
2.11. Western blots
Lung proteins separated by SDS polyacrylamide gel electrophoresis. The proteins transferred to polyvinylidene fluoride membranes, which incubated with specific antibodies overnight. Next, the membranes treated with secondary antibodies, then incubated with luminol/enhancer solution (Merck Millipore, Burlington. MA, USA) to present protein signals by BioSpectrum AC Imaging System (UVP, Upland, CA, USA). Specific antibodies included ICAM-1 (Abcam); lamin B1, Nrf2, and HO-1 (Santa Cruz, CA, USA); and β-actin (Sigma).
2.12. Real-time PCR analysis
TRIzol reagent solution (Life Technologies, Carlsbad, CA, USA) was used to extract RNA, and cDNA synthesized by the cDNA synthesis kit (Bio-Rad, San Francisco, CA, USA) [18]. Gene-specific cDNAs were amplified using a SYBR green master mix kit and the spectrofluorometric thermal cycler (iCycler; Bio-Rad) as described previously [18].
2.13. BEAS-2B cell and ginsenoside Rg3 treatment
BEAS-2B, human bronchial epithelial cells, (American Type Culture Collection, Manassas, VA, USA) were cultured in DMEM/F12 medium. Ginsenoside Rg3 was dissolved in 100% DMSO at a concentration of 100 mM. The DMSO content was ≤0.1% in all experimental media. BEAS-2B cells seeded on a 24-well plate, and treated with ginsenoside Rg3 (0–30 μM) for 1 h. Subsequently, cells treated with 10 ng/mL IL-4/TNF-α for 24 h. The supernatants were collected and chemokines or cytokines measured using specific ELISA kits.
2.14. ROS analysis
IL-4/TNF-α-induced BEAS-2B cells treated with ginsenoside Rg3 for 24 h. The cells incubated with 2′,7′-dichlorofluorescin diacetate (DCFH-DA) and ROS levels were then detected by use of a Multi-Mode microplate reader (SpectraMax i3X, Molecular Devices), as described previously [18]. Additionally, intracellular ROS was detected using a fluorescence microscope (Olympus, Tokyo, Japan).
2.15. Cell-cell adhesion analysis
BEAS-2B cells treated with or without ginsenoside Rg3 for 1 h, and then stimulated with 10 ng/mL IL-4/TNF-α for 24 h. THP-1 human monocytes were incubated with calcein-AM (Sigma) as described previously [18]. Subsequently, THP-1 cells co-cultured with BEAS-2B cells to observe THP-1 cell adhesion using a fluorescence microscope (Olympus).
2.16. Statistical analysis
The values are each presented as the mean ± SEM of at least three independent experiments. Statistical significance was determined using one-way analysis of variance followed by the Tukey-Kramer post hoc test. Data with values of p < 0.05 were considered to be statistically significant.
3. Results
3.1. Ginsenoside Rg3 suppressed eosinophil numbers in the BALF
Compared with the OVA-sensitized mice, asthmatic mice treated with prednisolone or ginsenoside Rg3 significantly decreased the numbers of eosinophils in BALF. We also found that ginsenoside Rg3 or prednisolone could significantly suppress the total number of cells in BALF when compared with the number in the OVA group mice (Fig. 1B).
3.2. Ginsenoside Rg3 attenuated AHR in mice
In mice that inhaled 40 mg/mL methacholine, treatment with ginsenoside Rg3 and prednisolone significantly attenuated Penh values when compared with the OVA group mice (Fig. 1C). The airway resistance of mice was measured by the forced intubation technique. In mice that inhaled 30 mg/mL methacholine, 10 mg/kg ginsenoside Rg3 and prednisolone significantly reduced airway resistance, in comparison with OVA-sensitized mice (Fig. 1D).
3.3. Ginsenoside Rg3 regulated chemokine and cytokine expressions in BALF and lung tissue
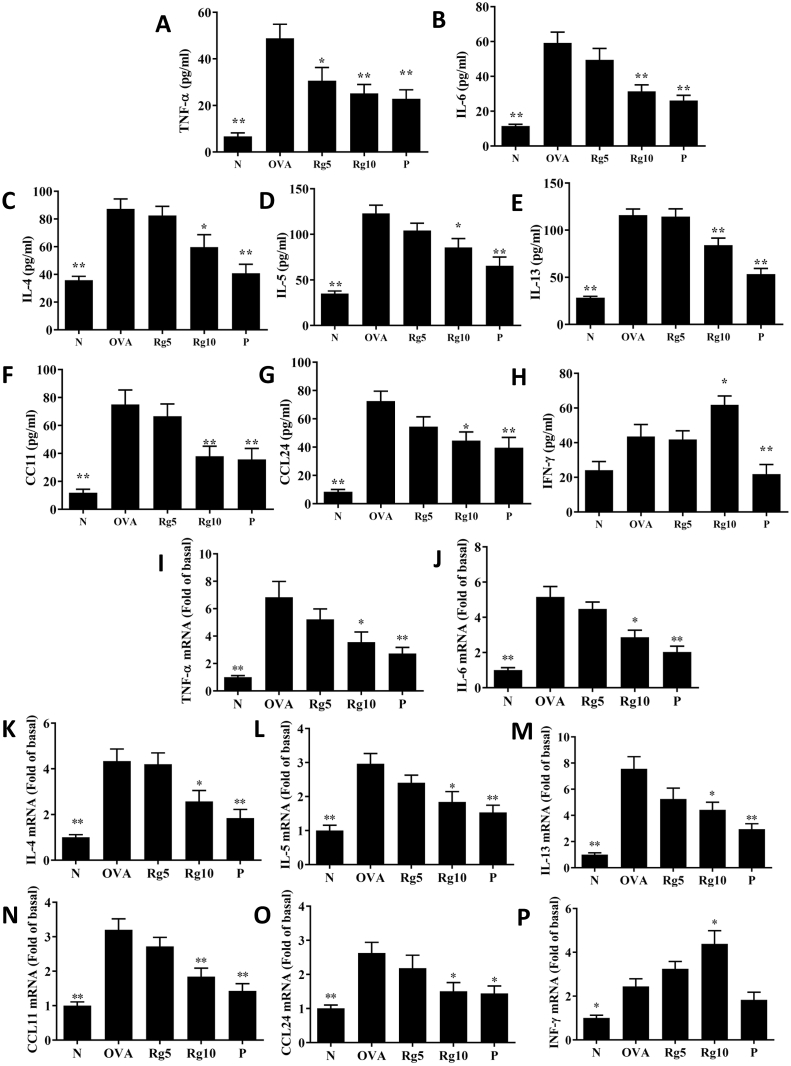
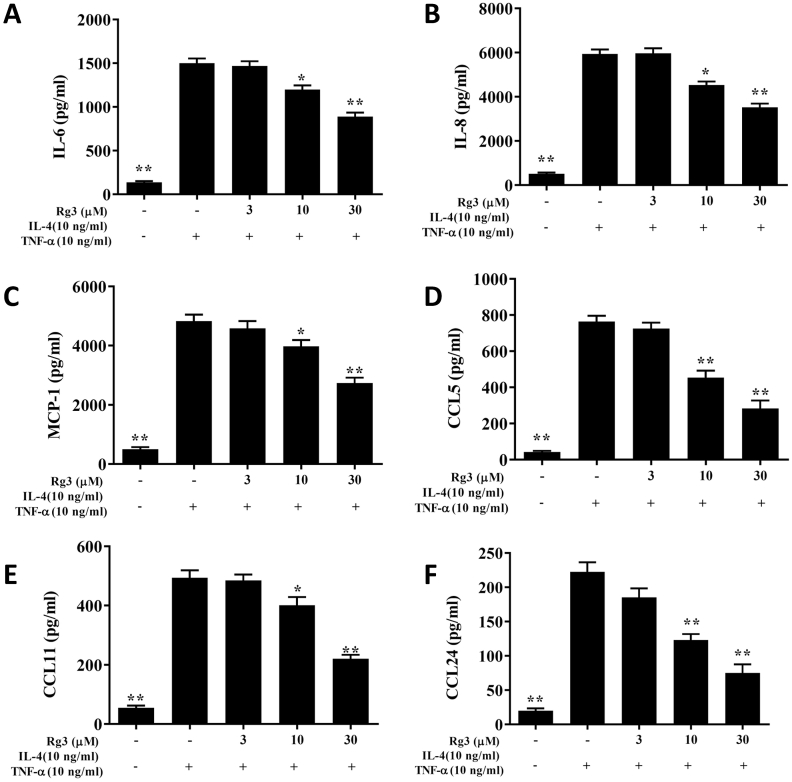
In BALF, ginsenoside Rg3 significantly decreased CCL11, CCL24, TNF-α, IL-5, IL-4, IL-13, and IL-6 levels in comparison with asthmatic mice (Fig. 2A–G). Treatment of asthmatic mice with ginsenoside Rg3 also significantly attenuated CCL11, CCL24, TNF-α, IL-4, IL-13, IL-5, and IL-6 gene expression in the lungs (Fig. 2I-O). Furthermore, in BALF and lung tissue, ginsenoside Rg3 significantly increased IFN-γ production over that in untreated asthmatic mice (Fig. 2H and P).
Fig. 2.
(A-H) Effects of ginsenoside Rg3 on the levels of cytokines and chemokines in BALF. (I–P) Gene expression of the lungs was determined using real-time RT-PCR with lung tissue from normal (N) and OVA-stimulated (OVA) mice with or without ginsenoside Rg3 (Rg5, Rg10) or prednisolone (P) treatment. Fold changes in expression were measured relative to β-actin (internal control). The data are presented as the mean ± SEM of three independent experiments (n = 10). ∗p < 0.05, ∗∗p < 0.01 compared to OVA control mice. 5 mg/kg and 10 mg/kg ginsenoside Rg3 were named as Rg5 and Rg10, respectively.
3.4. Ginsenoside Rg3 reduced OVA-induced goblet cell hyperplasia and eosinophil infiltration in lungs
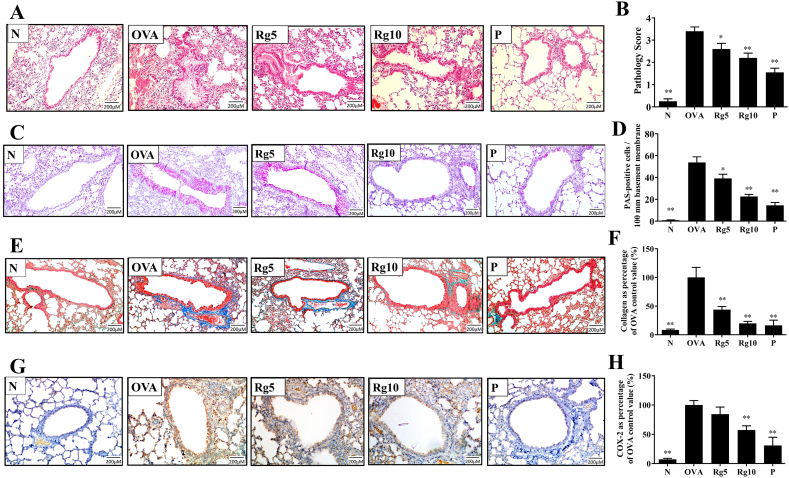
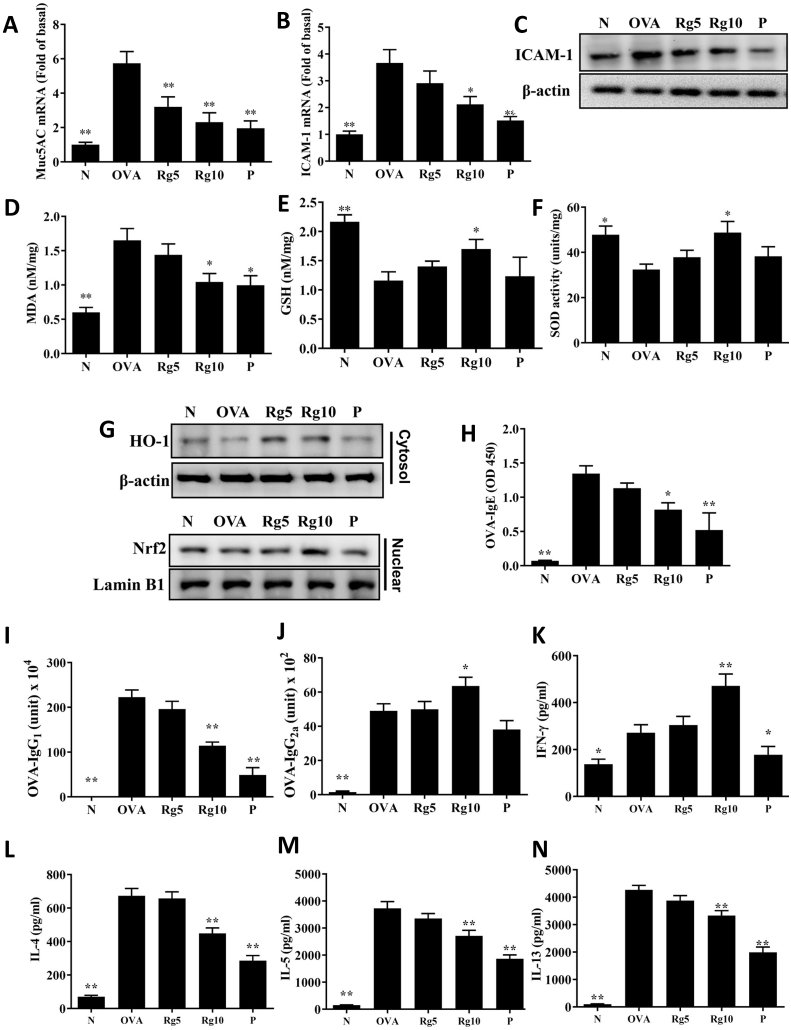
Asthmatic mice treated with ginsenoside Rg3 had reduced eosinophil infiltration and decreased tracheal goblet cell hyperplasia of the lungs compared with that of the OVA-sensitized mice (Fig. 3A–D). We also found that ginsenoside Rg3 reduced collagen production in the lung of asthmatic mice (Fig. 3E–F). Furthermore, Immunohistochemical staining of COX-2 in lung tissue showed that ginsenoside Rg3 treatment decreased COX-2 productions in comparison with the OVA group asthma mice (Fig. 3G–H). Moreover, ginsenoside Rg3 significantly decreased Muc5Ac and ICAM-1 gene expression, and reduced ICAM-1 protein expression in lung tissue, compared with their levels in untreated asthmatic mice (Fig. 4A–C).
Fig. 3.
Ginsenoside Rg3 effects on asthmatic lung tissue. Sections of lung tissue from normal (N) and OVA-stimulated (OVA) mice with or without ginsenoside Rg3 (Rg5, Rg10) or prednisolone (P) treatment. (A) Ginsenoside Rg3 reduced eosinophil infiltration (HE stain; 200 × magnification). (B) The pathological scores reflect the degree of eosinophil infiltration in lung tissue. (C) PAS-stained lung sections show goblet cell hyperplasia (200 × magnification). (D) The number of PAS-positive cells per 100 μm of basement membrane. (E) Lung sections were stained with Masson’s trichrome stain to detect collagen expression (200 × magnification). (F) Quantitative analysis of collagen in lung sections. (G) Ginsenoside Rg3 decreased COX-2 expression in the lungs. COX-2 expression was analyzed by immunohistochemistry (brown color). (H) Quantitative analysis of COX-2 expression in lung sections. Three independent experiments were analyzed, and all data are presented as the mean ± SEM. ∗p < 0.05 and ∗∗p < 0.01 compared with the OVA control group. 5 mg/kg and 10 mg/kg ginsenoside Rg3 were named as Rg5 and Rg10, respectively.
Fig. 4.
Real-time RT-PCR results showing gene expression levels of (A) Muc5AC and (B) ICAM-1 in lung tissue from normal (N) and OVA-stimulated (OVA) mice with or without ginsenoside Rg3 (Rg5, Rg10) or prednisolone (P) treatment. Fold changes in expression levels were calculated relative to that of β-actin (internal control). (C) Western blot showing that ginsenoside Rg3 suppressed ICAM-1 expression in lung tissue. Effects of ginsenoside Rg3 on oxidative stress factors. (D) MDA activity, (E) GSH activity, and (F) SOD activity were measured in mouse lung tissue. (G)Western blots show ginsenoside Rg3 modulation of Nrf2 and HO-1 expression in lung tissue. Furthermore, serum levels of (H) OVA-IgE, (I) OVA-IgG1, and (J) OVA-IgG2a in mice. Ginsenoside Rg3 modulated the levels of (K) IFN-γ, (L) IL-4, (M) IL-5, and (N) IL-13 produced by OVA-activated splenocytes. The data are presented as the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared with the OVA control group. 5 mg/kg and 10 mg/kg ginsenoside Rg3 were named as Rg5 and Rg10, respectively.
3.5. Ginsenoside Rg3 modulated MDA and GSH in the lungs
Compared with untreated OVA-sensitized mice, mice treated with ginsenoside Rg3 significantly increased GSH levels and SOD activity and lower MDA levels in their lung tissue (Fig. 4D–F). Ginsenoside Rg3-treatment of asthmatic mice promoted HO-1 expression and increased nuclear Nrf2 production in lung tissue (Fig. 4G).
3.6. Ginsenoside Rg3 modulated OVA-specific antibodies in serum and cytokine expressions in splenocyte
Ginsenoside Rg3 significantly decreased serum OVA-IgG1 and OVA-IgE and increased OVA-IgG2a expression in asthmatic mice (Fig. 4H–J). Furthermore, the supernatant of splenocytes from asthmatic mice treated with ginsenoside Rg3 showed reduced the levels of IL-13, IL-5, and IL-4 and increased IFN-γ expressions compared with the OVA group mice (Fig. 4K–N).
3.7. Ginsenoside Rg3 reduced inflammatory response and cell adhesion in BEAS-2B cells
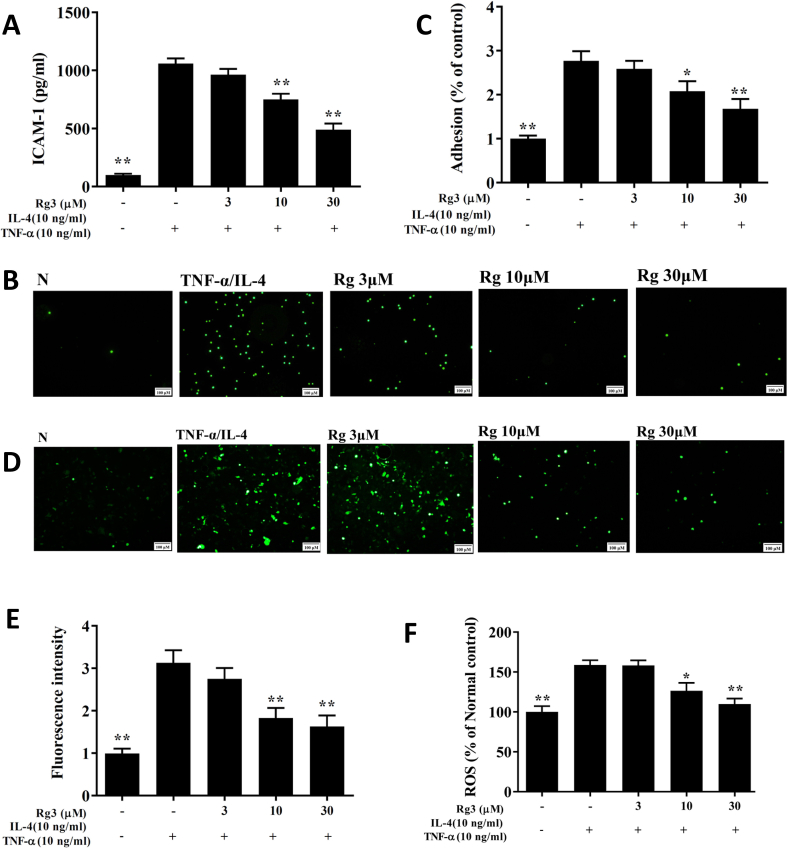
Ginsenoside Rg3-treated cells significantly decreased levels of CCL24, CCL11, CCL5, MCP-1, IL-8, and IL-6 compared with untreated activated BEAS-2B cells (Fig. 5). Ginsenoside Rg3-treated cells had decreased ICAM-1 levels (Fig. 6A), and were less adherent to THP-1 cells, compared with IL-4/TNF-αactivated BEAS-2B cells (Fig. 6B and C).
Fig. 5.
Effects of ginsenoside Rg3 (Rg3) on cytokine and chemokine production in BEAS-2B cells. ELISA showing (A) IL-6, (B) IL-8, (C) MCP-1, (D) CCL5, (E) CCL11, and (F) CCL24 levels in BEAS-2B cells treated with ginsenoside Rg3. The data are presented as the mean ± SEM of three independent experiments. ∗p < 0.05, ∗∗p < 0.01 compared to BEAS-2B cells stimulated with TNF-α and IL-4.
Fig. 6.
Ginsenoside Rg3 (Rg3) inhibited THP-1 cell adherence to activated BEAS-2B cells. (A) Ginsenoside Rg3 decreased the levels of ICAM-1 in BEAS-2B cells activated with TNF-α/IL-4. (B) Fluorescence microscope images of THP-1 cells labeled with calcein-AM and mixed with untreated cells (normal control, N) and TNF-α/IL-4-activated BEAS-2B cells in the absence or presence of ginsenoside Rg3. (C) Fluorescence intensity of monocytic cell adhesion to BEAS-2B cells. Ginsenoside Rg3 effects on ROS production in activated BEAS-2B cells. (D) Fluorescence microscopy images of intracellular ROS. (E) Fluorescence intensity of intracellular ROS. (F) Percentages of ROS detected in TNF-α/IL-4-activated BEAS-2B cells in the absence or presence of ginsenoside Rg3, compared with untreated cells (N). Three independent experiments were analyzed, and data represent the mean ± SEM. ∗p < 0.05, ∗∗p < 0.01, compared with BEAS-2B cells stimulated with TNF-α and IL-4.
3.8. Ginsenoside Rg3 suppressed ROS expression in BEAS-2B cells
Fluorescence microscopy revealed that ROS expression was lower in ginsenoside Rg3-treated, TNF-α/IL-4-activated BEAS-2B cells compared with untreated activated BEAS-2B cells (Fig. 6D and E). The DCFH-DA assay also showed that ginsenoside Rg3 treatment significantly attenuated ROS productions in IL-4/TNF-α activated BEAS-2B cells (Fig. 6F).
4. Discussion
In Chinese and Korean traditional medicine, the root of P. ginseng is commonly used to treat cardiovascular diseases and diabetes, promote immune function, and improve central nervous system function [10,28]. Ginsenoside Rg3 also reduces endothelial dysfunction caused by oxidative stress, by upregulating the Nrf2/HO-1 signaling pathway [29]. Ginsenoside Rg3 also inhibits COX-2 expression in IL-1β-activated lung epithelial cells, and decreases the levels of eotaxin, IL-13,IL-9, IL-4, and IL-6 in human asthmatic airway epithelial tissue [14]. However, how ginsenoside Rg3 ameliorates AHR, eosinophil infiltration, oxidative stress, and airway inflammation in the asthmatic lung was unclear. In the current study, ginsenoside Rg3 could suppress tracheal goblet cell hyperplasia, collagen expression, eosinophil infiltration, and AHR in the lungs of asthmatic mice. It also attenuated COX-2 expression and oxidative stress in the lung, and lowered Th2-associated cytokine and chemokine expressions in lung tissue and BALF. Furthermore, ginsenoside Rg3 inhibited ROS expression and decreased the secretion of chemokines and pro-inflammatory cytokines by inflammatory BEAS-2B cells.
AHR values can be used to detect airway flow and respiratory frequency, and to assess the patient’s respiratory pathological changes and respiratory system function [30]. Especially in patients with chronic asthma, the connective tissues of the lungs and airways gradually thicken and become weaker, reducing airway contractility and lowering alveolar surface tension [3]. Therefore, the patient’s intake of air during an emergency asthma attack is insufficient, resulting in an increased respiratory rate and shortness of breath. Our experiment found that ginsenoside Rg3 reduced both AHR and respiratory resistance in asthmatic mice. These results confirmed that ginsenoside Rg3 can improve respiratory function in asthmatic mice.
High levels of IL-13 can be detected in the lungs of asthmatic patients, and excess IL-13 secretion by Th2 cells worsens AHR [4]. Our experiments confirmed that asthmatic mice treated with ginsenoside Rg3 had decreased IL-13 levels in both lung and BALF, and AHR was also found to be significantly lower in ginsenoside Rg3-treated asthmatic mice. These results suggest that ginsenoside Rg3 inhibited AHR in asthmatic mice mainly by blocking the expression of IL-13. The lungs of most asthma patients are infiltrated by large numbers of eosinophils, and activated Th2 cells secrete excess IL-5, inducing the differentiation of more mature eosinophils from bone marrow [31]. Tracheal epithelial cells release high amounts of eotaxin under the induction of IL-4 and TNF-α, and human lung epithelial cells also release CCL26 in response to IL-4 [18,32]. These eotaxins attract more eosinophils to the airways and lungs in asthmatic patients [4]. Activated eosinophils undergo degranulation and release cytotoxic molecules causing edema and inflammation of the airways and tracheal epithelial cells [33,34]. Previous studies demonstrated that ginsenoside Rg3 significantly inhibits mast cell histamine release and reduces allergic inflammation [35]. Our experiments showed that ginsenoside Rg3 treatment of asthmatic mice not only inhibited IL-4 and IL-5 expression in BALF, lung, and splenocytes stimulated by OVA, but also decreased serum OVA-IgG1 and OVA-IgE levels. In addition, ginsenoside Rg3 reduced the releases of eotaxin (CCL11 and CCL24) from tracheal epithelial cells and decreased the expression of ICAM-1, blocking immune cell adhesion to tracheal epithelial cells. In the lungs of patients with asthma, Th2 cells secrete excess IL-4, IL-5, and IL-13 to inhibit the expression of Th1 cells, which reduces IFN-γ production [5]. The IgG2a and IgG1 immunoglobulin were confirmed as markers for Th1 and Th2 cells, respectively [4]. We therefore detected the expression of IFN-γ, a Th1-polarizing cytokine. Ginsenoside Rg3 could increase IFN-γ levels in the BALF and spleen cell supernatants. We believe that ginsenoside Rg3 suppressed the development of asthma by suppressing Th2 cell activity and reducing eotaxin secretion in asthmatic mice, thereby inhibiting eosinophilic infiltration into the lung tissue.
Allergens stimulate the differentiation of airway epithelial cells to goblet cells, which then secrete excess mucus, and goblet cell proliferation is one of the characteristics of airway remodeling in asthmatic patients [2]. Muc5AC gene expression in the lung and goblet cell proliferation in the airways do not increase in response to an allergen in IL-13 or IL-4 knockout asthmatic mice [36]. P. ginseng extract can ameliorate Muc5AC gene expression in asthma mice [37]. In our experiments, ginsenoside Rg3 treatment reduced Muc5AC gene expression in the lungs of asthmatic mice and also attenuated airway goblet cell proliferation, both of which improve breathing. We believe ginsenoside Rg3 achieves these effects by reducing the expression of Il-4 and IL-13 in asthmatic mice.
Chronic inflammation of the lungs causes pulmonary fibrosis characterized by collagen deposition, persistent inflammation, and oxidative damage to lung epithelial cells [2]. Fibrotic alveolar walls harden and lose elasticity, reducing vital capacity and impeding oxygen inhalation and carbon dioxide exhalation [2]. Inflammatory eosinophils release osteopontin to induce airway fibrosis through promoted Th2 cell activity [38]. Total ginsenoside suppressed pulmonary fibrosis in mice by regulating the matrix metalloproteinase system and TGF-β1/Smad signaling pathway [39]. Here, ginsenoside Rg3 treatment significantly reduced the expression of the proinflammatory cytokines IL-6 and TNF-α in the lung, thereby suppressing collagen deposition and reducing pulmonary fibrosis in asthmatic mice.
Allergens or microorganisms stimulate respiratory epithelial cells, which induce inflammation and activate epithelial cells to release inflammatory cytokines causing inflammation in the airways and lungs [6]. These inflammatory epithelial cells also release MCP-1, CCL5, and IL-8 to attract more macrophages and neutrophils to the lungs [40]. These activated immune cells release more inflammatory cytokines and inflammatory mediators, which exacerbate cell damage and fibrosis in the lungs [41]. Here, we found that ginsenoside Rg3 not only suppressed the release of CCL5, MCP-1, IL-8, and IL-6 by inflammatory BEAS-2B cells, but also decreased the inflammatory cytokine and chemokine expressions in BALF and the lung. Immunohistochemical staining showed that ginsenoside Rg3 reduced the expression of COX-2 in the lungs of asthmatic mice. Thus, ginsenoside Rg3 could significantly reduce lung inflammation and pulmonary cachexia by reducing alveolar cell damage in asthmatic mice.
Sudden or persistent oxidative stress in the lungs induces sputum production and damages alveolar cells or tracheal epithelial cells in patients with asthma [42]. Inflammatory immune cells infiltrating the lungs release more oxidative stress, and stimulate respiratory epithelial cells to release more inflammatory cytokines and ROS [43]. Activated eosinophils release eosinophil peroxidase and cause oxidative damage in asthmatic patients [44]. Oxidative stress exacerbates bronchoconstriction, stimulates mucus secretion, increases AHR, and causes shortness of breath and difficulty breathing [42]. Ginsenoside Rg3 increases Nrf2/HO-1 expression and thereby attenuates oxidative stress in endothelial cells [29]. In our experiments, ginsenoside Rg3 significantly increased the expression of Nrf2, HO-1, SOD, and GSH, and reduced MDA levels in the lungs of asthmatic mice. Collectively, our experiments confirmed that ginsenoside Rg3 is an excellent stimulator of antioxidant activity and can attenuate allergen-induced lung cell damage in asthmatic mice.
In conclusion, ginsenoside Rg3 significantly suppressed oxidative stress and inflammation in the lungs of asthmatic mice. It also decreased AHR, eosinophil infiltration, and mucus hypersecretion by inhibiting Th2 cytokine and eotaxin expressions. We suggest that ginsenoside Rg3 has extremely good potential as a regulator in reducing anti-oxidative stress and inflammation in asthma.
Author contributions
WCH, THH, KWY, YLC, and CJL performed and designed the experiments; KWY, YLC, and SCS interpreted and analyzed the experimental data; WCH, THH, and CJL drafted the manuscript.
Declaration of competing interest
The authors declare that there are no conflicts of interest.
Acknowledgments
This study supported by grants from Chang Gung Memorial Hospital in Taiwan (CMRPF1K0051, CMRPF1J0062, CMRPF1H0022, CMRPF1H0042, and CMRPF1J0021) and from the Ministry of Science and Technology in Taiwan (106-2320-B-255-008-MY3 and 106-2320-B-255-007-MY3).
References
- 1.Chen X., Corry D.B., Li E. Mechanisms of allergy and adult asthma. Curr Opin Allergy Clin Immunol. 2020;20:36–42. doi: 10.1097/ACI.0000000000000601. [DOI] [PubMed] [Google Scholar]
- 2.Kudo M., Ishigatsubo Y., Aoki I. Pathology of asthma. Front Microbiol. 2013;4:263. doi: 10.3389/fmicb.2013.00263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ray A., Raundhal M., Oriss T.B., Ray P., Wenzel S.E. Current concepts of severe asthma. J Clin Invest. 2016;126:2394–2403. doi: 10.1172/JCI84144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lambrecht B.N., Hammad H., Fahy J.V. The cytokines of asthma. Immunity. 2019;50:975–991. doi: 10.1016/j.immuni.2019.03.018. [DOI] [PubMed] [Google Scholar]
- 5.Caminati M., Pham D.L., Bagnasco D., Canonica G.W. Type 2 immunity in asthma. World Allergy Organ J. 2018;11:13. doi: 10.1186/s40413-018-0192-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roan F., Obata-Ninomiya K., Ziegler S.F. Epithelial cell-derived cytokines: more than just signaling the alarm. J Clin Invest. 2019;129:1441–1451. doi: 10.1172/JCI124606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goleva E., Berdyshev E., Leung D.Y. Epithelial barrier repair and prevention of allergy. J Clin Invest. 2019;129:1463–1474. doi: 10.1172/JCI124608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Choi M.K., Song I.S. Interactions of ginseng with therapeutic drugs. Arch Pharm Res. 2019;42:862–878. doi: 10.1007/s12272-019-01184-3. [DOI] [PubMed] [Google Scholar]
- 9.Lim C.Y., Moon J.M., Kim B.Y., Lim S.H., Lee G.S., Yu H.S. Comparative study of Korean White Ginseng and Korean Red Ginseng on efficacies of OVA-induced asthma model in mice. J Ginseng Res. 2015;39:38–45. doi: 10.1016/j.jgr.2014.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J.H. Pharmacological and medical applications of Panax ginseng and ginsenosides: a review for use in cardiovascular diseases. J Ginseng Res. 2018;42:264–269. doi: 10.1016/j.jgr.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mohanan P., Subramaniyam S., Mathiyalagan R., Yang D.C. Molecular signaling of ginsenosides Rb1, Rg1, and Rg3 and their mode of actions. J Ginseng Res. 2018;42:123–132. doi: 10.1016/j.jgr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Z., Li L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-kappaB (NF-kappaB) signaling pathway. Int Immunopharmacol. 2016;34:53–59. doi: 10.1016/j.intimp.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 13.Yang J., Li S., Wang L., Du F., Zhou X., Song Q. Ginsenoside Rg3 attenuates lipopolysaccharide-induced acute lung injury via MerTK-dependent activation of the PI3K/AKT/mTOR pathway. Front Pharmacol. 2018;9:850. doi: 10.3389/fphar.2018.00850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee I.S., Uh I., Kim K.S., Kim K.H., Park J., Kim Y. Anti-inflammatory effects of ginsenoside Rg3 via NF-kappaB pathway in A549 cells and human asthmatic lung tissue. J Immunol Res. 2016;2016:7521601. doi: 10.1155/2016/7521601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen T., Xiao L., Zhu L., Ma S., Yan T., Ji H. Anti-asthmatic effects of Ginsenoside Rb1 in a mouse model of allergic asthma through relegating Th1/Th2. Inflammation. 2015;38:1814–1822. doi: 10.1007/s10753-015-0159-4. [DOI] [PubMed] [Google Scholar]
- 16.Li L.C., Piao H.M., Zheng M.Y., Lin Z.H., Choi Y.H., Yan G.H. Ginsenoside Rh2 attenuates allergic airway inflammation by modulating nuclear factor-kappaB activation in a murine model of asthma. Mol Med Rep. 2015;12:6946–6954. doi: 10.3892/mmr.2015.4272. [DOI] [PubMed] [Google Scholar]
- 17.Cheng Z., Li L. Ginsenoside Rg3 ameliorates lipopolysaccharide-induced acute lung injury in mice through inactivating the nuclear factor-κB (NF-κB) signaling pathway. Int Immunopharmacol. 2016;34:53–59. doi: 10.1016/j.intimp.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 18.Huang W.C., Fang L.W., Liou C.J. Phloretin attenuates allergic airway inflammation and oxidative stress in asthmatic mice. Front Immunol. 2017;8:134. doi: 10.3389/fimmu.2017.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huang W.C., Liu C.Y., Shen S.C., Chen L.C., Yeh K.W., Liu S.H. Protective effects of licochalcone A improve airway hyper-responsiveness and oxidative stress in a mouse model of asthma. Cells. 2019;8:617. doi: 10.3390/cells8060617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liou C.J., Huan Y.L., Huang W.C., Yeh K.W., Huang T.Y., Lin C.F. Water extract of Helminthostachys zeylanica attenuates LPS-induced acute lung injury in mice by modulating NF-κB and MAPK pathways. J Ethnopharmacol. 2017;199:30–38. doi: 10.1016/j.jep.2017.01.043. [DOI] [PubMed] [Google Scholar]
- 21.Liou C.J., Chen Y.L., Yu M.C., Yeh K.W., Shen S.C., Huang W.C. Sesamol alleviates airway hyperresponsiveness and oxidative stress in asthmatic mice. Antioxidants (Basel) 2020;9 doi: 10.3390/antiox9040295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang W.C., Chan C.C., Wu S.J., Chen L.C., Shen J.J., Kuo M.L. Matrine attenuates allergic airway inflammation and eosinophil infiltration by suppressing eotaxin and Th2 cytokine production in asthmatic mice. J Ethnopharmacol. 2014;151:470–477. doi: 10.1016/j.jep.2013.10.065. [DOI] [PubMed] [Google Scholar]
- 23.Liou C.J., Cheng C.Y., Yeh K.W., Wu Y.H., Huang W.C. Protective effects of casticin from Vitex trifolia alleviate eosinophilic airway inflammation and oxidative stress in a murine asthma model. Front Pharmacol. 2018;9:635. doi: 10.3389/fphar.2018.00635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liou C.J., Huang W.C., Kuo M.L., Yang R.C., Shen J.J. Long-term oral administration of Gynostemma pentaphyllum extract attenuates airway inflammation and Th2 cell activities in ovalbumin-sensitized mice. Food Chem Toxicol. 2010;48:2592–2598. doi: 10.1016/j.fct.2010.06.020. [DOI] [PubMed] [Google Scholar]
- 25.Myou S., Leff A.R., Myo S., Boetticher E., Tong J., Meliton A.Y. Blockade of inflammation and airway hyperresponsiveness in immune-sensitized mice by dominant-negative phosphoinositide 3-kinase-TAT. J Exp Med. 2003;198:1573–1582. doi: 10.1084/jem.20030298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou C.J., Huang W.C. Dehydroepiandrosterone suppresses eosinophil infiltration and airway hyperresponsiveness via modulation of chemokines and Th2 cytokines in ovalbumin-sensitized mice. J Clin Immunol. 2011;31:656–665. doi: 10.1007/s10875-011-9529-3. [DOI] [PubMed] [Google Scholar]
- 27.Huang W.C., Ting N.C., Huang Y.L., Chen L.C., Lin C.F., Liou C.J. Helminthostachys zeylanica water extract ameliorates airway hyperresponsiveness and eosinophil infiltration by reducing oxidative stress and Th2 cytokine production in a mouse asthma model. Mediators Inflamm. 2020;2020:1702935. doi: 10.1155/2020/1702935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen W., Balan P., Popovich D.G. Review of ginseng anti-diabetic studies. Molecules. 2019;24 doi: 10.3390/molecules24244501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Chen L., Wang T., Jiang X., Zhang H., Li P. Ginsenoside Rg3 antagonizes adriamycin-induced cardiotoxicity by improving endothelial dysfunction from oxidative stress via upregulating the Nrf2-ARE pathway through the activation of akt. Phytomedicine. 2015;22:875–884. doi: 10.1016/j.phymed.2015.06.010. [DOI] [PubMed] [Google Scholar]
- 30.Saeki M., Nishimura T., Kitamura N., Hiroi T., Mori A., Kaminuma O. Potential mechanisms of T cell-mediated and eosinophil-independent bronchial hyperresponsiveness. Int J Mol Sci. 2019;20:2980. doi: 10.3390/ijms20122980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pelaia C., Paoletti G., Puggioni F., Racca F., Pelaia G., Canonica G.W. Interleukin-5 in the pathophysiology of severe asthma. Front Physiol. 2019;10:1514. doi: 10.3389/fphys.2019.01514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Coleman S.L., Kruger M.C., Sawyer G.M., Hurst R.D. Procyanidin A2 modulates IL-4-induced CCL26 production in human alveolar epithelial cells. Int J Mol Sci. 2016;17:1888. doi: 10.3390/ijms17111888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yancey S.W., Keene O.N., Albers F.C., Ortega H., Bates S., Bleecker E.R. Biomarkers for severe eosinophilic asthma. J Allergy Clin Immunol. 2017;140:1509–1518. doi: 10.1016/j.jaci.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 34.Weissler J.C. Eosinophilic lung disease. Am J Med Sci. 2017;354:339–349. doi: 10.1016/j.amjms.2017.03.020. [DOI] [PubMed] [Google Scholar]
- 35.Kee J.Y., Hong S.H. Ginsenoside Rg3 suppresses mast cell-mediated allergic inflammation via mitogen-activated protein kinase signaling pathway. J Ginseng Res. 2019;43:282–290. doi: 10.1016/j.jgr.2018.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Oeser K., Maxeiner J., Symowski C., Stassen M., Voehringer D. T cells are the critical source of IL-4/IL-13 in a mouse model of allergic asthma. Allergy. 2015;70:1440–1449. doi: 10.1111/all.12705. [DOI] [PubMed] [Google Scholar]
- 37.Kim D.Y., Yang W.M. Panax ginseng ameliorates airway inflammation in an ovalbumin-sensitized mouse allergic asthma model. J Ethnopharmacol. 2011;136:230–235. doi: 10.1016/j.jep.2011.04.048. [DOI] [PubMed] [Google Scholar]
- 38.Hirahara K., Aoki A., Morimoto Y., Kiuchi M., Okano M., Nakayama T. The immunopathology of lung fibrosis: amphiregulin-producing pathogenic memory T helper-2 cells control the airway fibrotic responses by inducing eosinophils to secrete osteopontin. Semin Immunopathol. 2019;41:339–348. doi: 10.1007/s00281-019-00735-6. [DOI] [PubMed] [Google Scholar]
- 39.Yang L., Chen P.P., Luo M., Shi W.L., Hou D.S., Gao Y. Inhibitory effects of total ginsenoside on bleomycin-induced pulmonary fibrosis in mice. Biomed Pharmacother. 2019;114:108851. doi: 10.1016/j.biopha.2019.108851. [DOI] [PubMed] [Google Scholar]
- 40.Liu C., Zhang X., Xiang Y., Qu X., Liu H., Tan M. Role of epithelial chemokines in the pathogenesis of airway inflammation in asthma (Review) Mol Med Rep. 2018;17:6935–6941. doi: 10.3892/mmr.2018.8739. [DOI] [PubMed] [Google Scholar]
- 41.Bush A. Cytokines and chemokines as biomarkers of future asthma. Front Pediatr. 2019;7:72. doi: 10.3389/fped.2019.00072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Antus B. Oxidative stress markers in sputum. Oxid Med Cell Longev. 2016;2016:2930434. doi: 10.1155/2016/2930434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Menzel M., Ramu S., Calven J., Olejnicka B., Sverrild A., Porsbjerg C. Oxidative stress attenuates TLR3 responsiveness and impairs anti-viral mechanisms in bronchial epithelial cells from COPD and asthma patients. Front Immunol. 2019;10:2765. doi: 10.3389/fimmu.2019.02765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Drake M.G., Lebold K.M., Roth-Carter Q.R., Pincus A.B., Blum E.D., Proskocil B.J. Eosinophil and airway nerve interactions in asthma. J Leukoc Biol. 2018;104:61–67. doi: 10.1002/JLB.3MR1117-426R. [DOI] [PMC free article] [PubMed] [Google Scholar]