Figure 4.
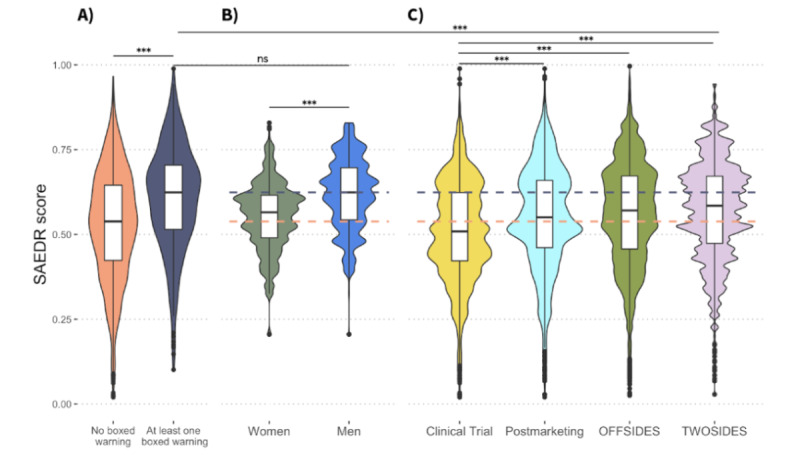
Differences in adverse drug reaction (ADR) severity between ADR groupings and discovery periods: ADR groups (x-axis) versus SAEDR scores (y-axis). The grey dashed line indicates the median severity, 0.624, of the ADRs that have been included in a boxed warning. The orange boxed line indicates the median severity, 0.538, of the ADRs that appear on a drug label but have not been included in a boxed warning. (A) The ADRs that were listed as a black box warning at least once (n=356) were significantly more severe than those that have not appeared in a black box warning (n=3305). (B) The ADRs that are disproportionately reported for men (n=56,405) are significantly more severe than those disproportionately reported for women (n=50,801). There was no significant difference (ns) in severity between the ADRs included in black box warnings and those disproportionately reported for men. (C) The ADRs discovered in the postmarketing period (n=11,506) are significantly more severe (*** indicate P<.001) than those discovered in the clinical trials (n=35,450). The ADRs identified in the postmarketing period through OFFSIDES (n=350,631) and the postmarketing polypharmacy ADRs identified through TWOSIDES (n=4,210,513) are significantly more severe than those discovered in the clinical trials. The severity of all postmarketing ADR groups is significantly less than the severity of the ADRs that have appeared in a black box warning. SAEDR: Severity of Adverse Events Derived from Reddit.
