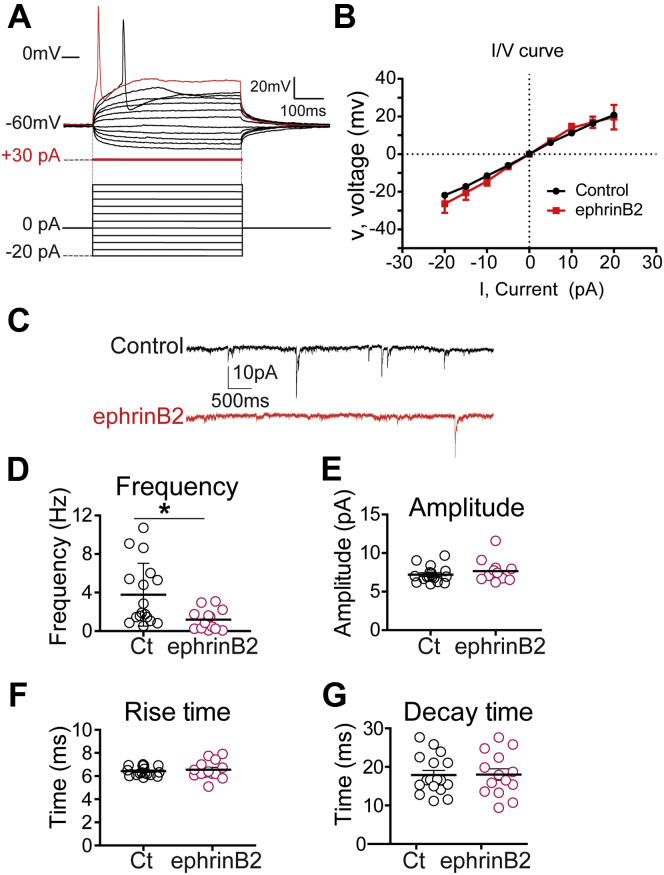
Figure 6.
EphB2 controls synaptic inputs in enteric neurons.A–G, primary cultures of ENS were grown for 12 DIV. Cultures where then stimulated with control (Ct) or ephrinB2 for 5 consecutive days. A, characteristic membrane properties and spiking pattern of primary enteric neurons obtained with a KCl-based intracellular solution. Raw traces show individual voltage responses to series of 500 ms current pulses from −20 pA to +30 pA with 5 pA steps (black traces) and to +30 pA above AP threshold (red traces). B, enteric neurons steady-state average I/V relationship from neurons recorded with a KCl-based internal solution (red curve: ephrinB2; black curve: control). Passive and active membrane properties of enteric neurons recorded in current-clamp configuration using a KCl-based intracellular solution are summarized in Table 1. C, representative traces of mPSCs recorded from enteric neurons stimulated with Control-Fc (Control) or ephrinB2-Fc (ephrinB2). D, quantification of mPSC frequency, meanControl versus ephrinB2: 3.781 ± 0.79 Hz versus 1.184 ± 0.3 Hz; p = 0.0136, n = 13–17 neurons per condition, Mann-Whitney test, Control/ephrinB2: U = 52. E, amplitude, meanControl versus ephrinB2: 7.195 ± 0.25 pA versus 7.647 ± 0.39 pA; p = 0.3003, Mann–Whitney test, Control/ephrinB2: U = 85. F, rise time, meanControl versus ephrinB2: 6.420 ± 0.1 ms versus 6.542 ± 0.21 ms, p = 0.7101, Mann–Whitney test, Control/ephrinB2: U = 109. G, decay time, meanControl versus ephrinB2: 17.88 ± 1.2 ms versus 18 ± 1.49 ms; p = 0.9844, Mann–Whitney test, Control/ephrinB2: U = 118. For all recordings, n = 13–17 neurons from at least five independent cultures, nonparametric Mann–Whitney test; ∗p < 0.05.