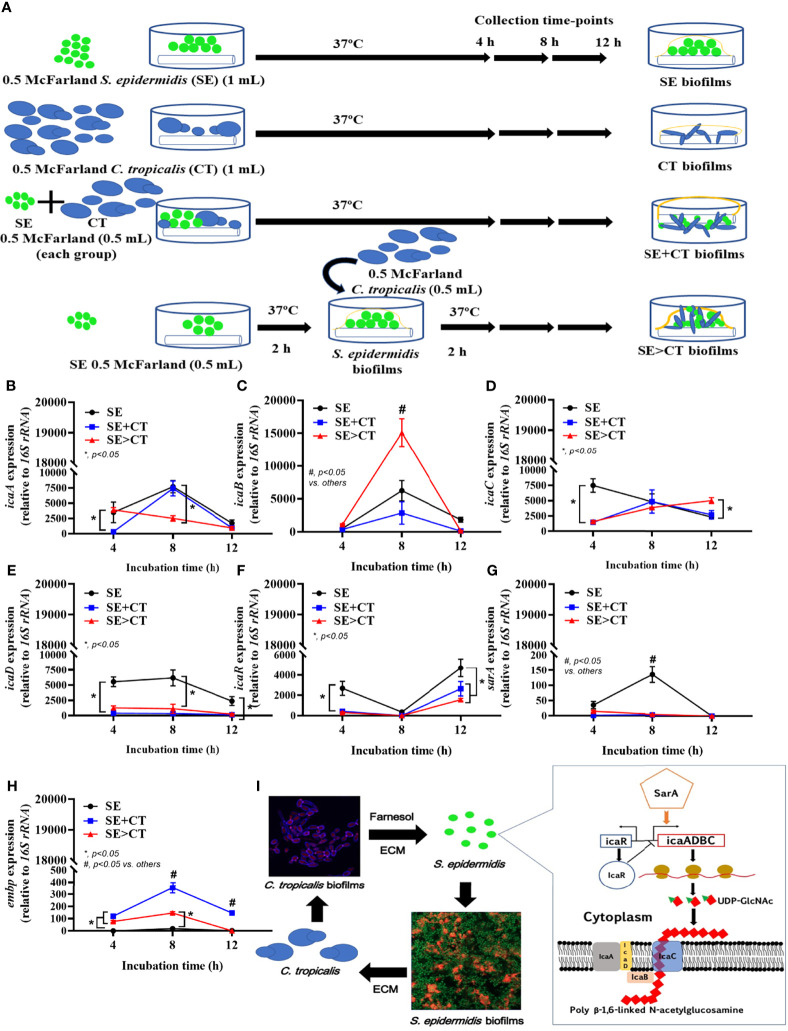
Figure 4.
The representative pictures of biofilm thickness by the lateral view of z-stack fluorescent stained pictures from clinically isolated Staphylococcus epidermidis (SE) and Candida tropicalis (CT) mixing (SE + CT) (left side) and SE before CT (SE > CT) (right side), indicating bacterial nucleic acid (SYTO9; green color fluorescence), extracellular matrix (wheat germ agglutinin (WAG)-AF647; red color fluorescence), and fungal cell wall (CW2MR; blue-purple color fluorescence) (A) are shown. Expression of SE biofilm-associated genes from cover-glass biofilms of clinically isolated SE (SE), Candida tropicalis (CT), SE simultaneously mixed with CT (SE + CT), and preformed SE biofilms following by CT (SE > CT) in different time-points (B–G) are demonstrated (independent triplicate experiments were performed). Diagram of the possible linkage between CT and SE biofilm synthesis indicates that Candida-producing farnesol, a quorum-sensing molecule blocking the yeast-to-filament conversion in fungal biofilms (Carolus et al., 2019), stimulates SE biofilm synthesis through the upregulated sarA and downregulated icaR (as demonstrated in the pathway) (Brescó et al., 2017) (H). Notably, icaA and icaD are responsible for UDP-Glc-NAc [uridine diphosphate N-acetylglucosamine, a single unit of the polysaccharide intercellular adhesin (PIA) in extracellular matrix (ECM)], while icaB and icaC are used for polymerization and transportation of ECM, respectively (Arciola et al., 2015) and the possible association with Candida (I). Meanwhile, embp expression increases bacterial adhesive protein of ECM. The results were from three independent experiments in triplicate as the mean ± SEM; p < 0.05 was considered statistically significant.