Table 1. Rate Constants for Desorption and Diffusion of Ammonia on Platinuma,b.
| elementary rate constants | fitted parameters | fit results |
|---|---|---|
| kdT(T) | Ea,dT/eV | 1.09 ± 0.02 |
| log10(AdT/s–1) | 14.8 ± 0.2 | |
| khS(T) | ΔEST/eVc | 0.23 ± 0.03 |
| log10(AhS/s–1) | 13.7 ± 0.6 | |
| khT(T) | Ea,hT/eV | 0.73 ± 0.04 |
| log10(AhT/s–1) | 13.6 ± 0.4 | |
| derived quantities | ||
| kdS(T) = kdkhS/kh | Ea,dS/eV | 1.32 ± 0.04 |
| log10(AdS/s–1) | 14.9 ± 0.6 | |
| DT(T)d | log10(D0T/cm2 s–1) | –1.9 ± 0.4 |
Results were obtained from the global fit of the kinetics model to experimental desorption rates from Pt(111) and Pt(332).
The elementary rate constants are parametrized according to the Arrhenius equation: k(T) = A exp(−Ea/kBT).
Ea,hS = Ea,h + ΔEST. Since AhS ≈ Ah, the difference of activation energies ΔEST is nearly equal to the difference of binding energies ΔE0, ST.
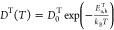 , where D0T is derived from Ah following
ref (29).
, where D0T is derived from Ah following
ref (29).
