Figure 1. General steps of a typical LC-MS/MS experiment.
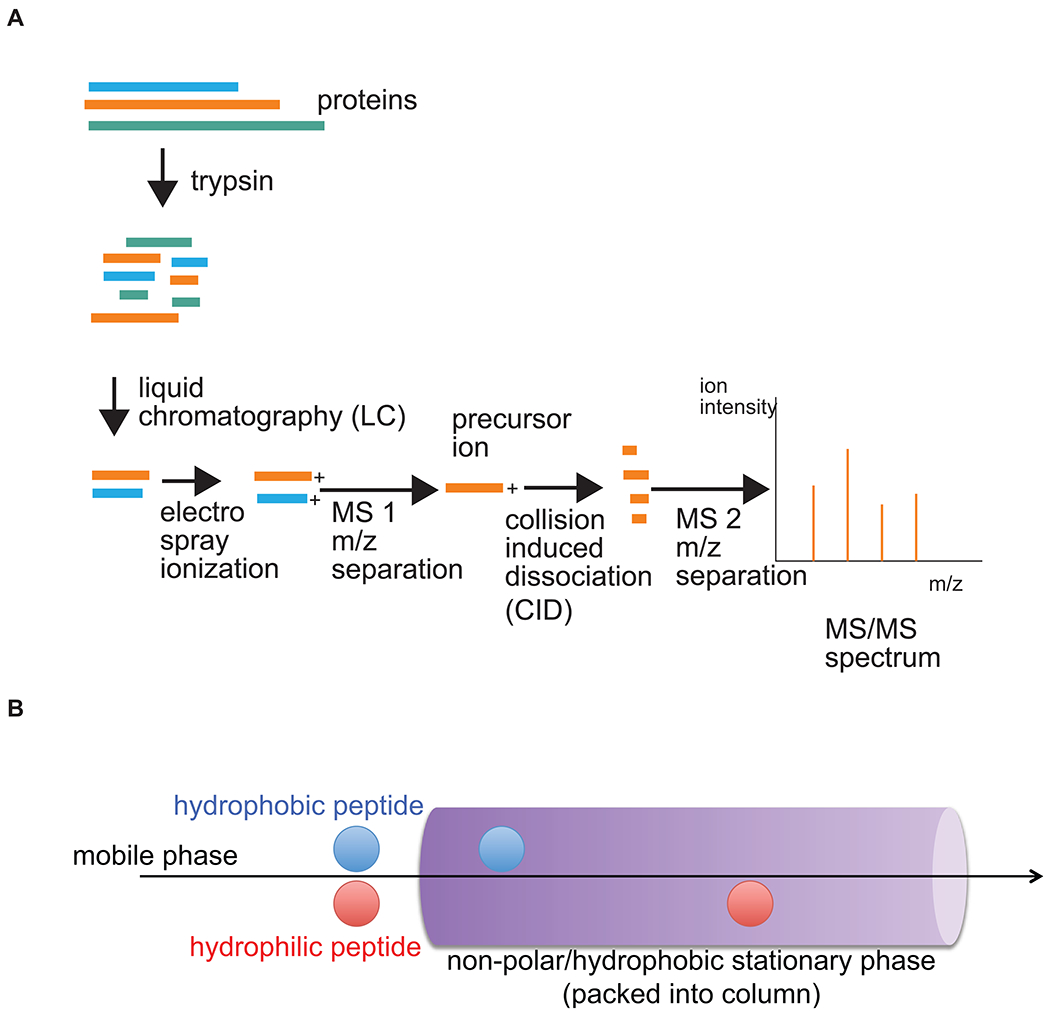
A) After isolation during the experiment of interest, proteins are treated with proteolytic enzymes (e.g. trypsin), then subjected to liquid chromatography (LC; explained in B)). Separated peptides are then ionized (i.e. by exposing drops of peptide-containing eluate from LC to a strong electric field, an atomic gas is formed) and separated by their mass (m)-to-charge (z) ratios in the first mass spectrometer (MS1). Precursor ions of a given m/z are then further fragmented by collision-induced dissociation (CID) and the ion fragments separated again (MS2). Resulting fragment ion spectra are recorded and analyzed as detailed in the text. The basic principle of reverse-phase LC is illustrated in B); the most hydrophobic peptides interact best with the non-polar stationary phase, whereas the least hydrophobic components elute first. Complete elution off the column, including the most non-polar peptides, is ensured by gradually increasing the concentration of non-polar solvents in the mobile phase.
