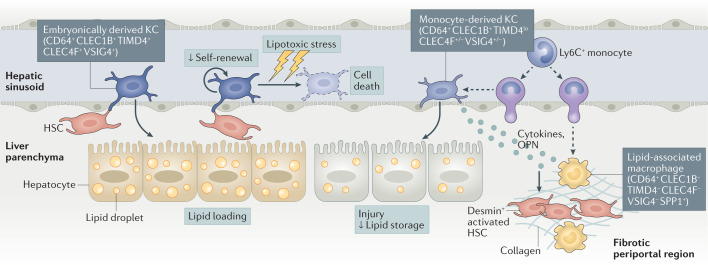
Fig. 4. The diversity of hepatic macrophages during NASH.
During non-alcoholic steatohepatitis (NASH), embryonically derived Kupffer cells (KCs), which are tissue-resident macrophages that inhabit the sinusoids of the healthy liver, are gradually lost. In response to the NASH environment, they lose their capacity to self-renew and show accelerated signs of cell death owing to lipotoxic stress. To maintain the KC pool, circulating Ly6C+ monocytes are recruited to the liver sinusoids, where they differentiate into monocyte-derived KCs. These monocyte-derived KCs express the pan-macrophage marker CD64 and have high levels of expression of CLEC1B (the prototypical marker of embryonically derived KCs) but lower levels of expression of other markers of embryonically derived KCs (such as CLEC4F, VSIG4 and TIMD4). Monocyte-derived KCs are more pro-inflammatory than embryonically derived KCs and they affect the liver response to NASH by limiting liver lipid storage and promoting liver injury. Other macrophages, also derived from circulating monocytes, populate the liver during NASH. They express no typical markers of KCs and are prevalent in fibrotic areas positive for desmin, a marker of activated hepatic stellate cells (HSCs). These monocyte-derived macrophages have been termed lipid-associated macrophages and they resemble the TREM2+CD9+ scar-associated macrophages that have been described in human fibrotic livers. Lipid-associated macrophages and monocyte-derived KCs express high levels of SPP1, the gene encoding osteopontin (OPN), which is a strong inducer of liver fibrogenesis.