Table 2.
Small molecule inhibitors targeting glutaminolysis pathway.
| Small Molecule Inhibitors Targeting Glutaminolysis Pathway | |||||
|---|---|---|---|---|---|
|
| |||||
| Small Molecule Inhibitors | Chemical Structure | Inhibition Type | Enzyme Target | Inhibitory Activity | Reference |
|
| |||||
| 6-diazo-5-oxo-l-norleucine (DON) |
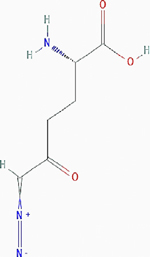
|
Irreversible inhibition | Nonselective. Glutamine utilizing enzymes, including glutamine amidotransferases | Inhibits the enzymes by covalently modifying the cysteine residues in the glutamine active sites irreversibly. | PubChem CID: 9087 URL: https://pubchem.ncbi.nlm.nih.gov/compound/9087#section=2D-Structure [38,40,90] |
| Acivicin |
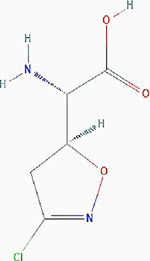
|
Irreversible inhibition | Nonselective. Glutamine utilizing enzymes, including glutamine amidotransferases, γ-glutamyltranspeptidase (GGT) | Inhibits the enzymes by forming an imine–thioether adduct at the cysteine residue of the active site as a result of nucleophilic substitution of the chlorine atom. Inhibits GGT by covalently modifying the Thr391 residue at the active site. |
PubChem CID: 294641 URL: https://pubchem.ncbi.nlm.nih.gov/compound/294641#section=2D-Structure [40,91,92] |
| Azaserine |
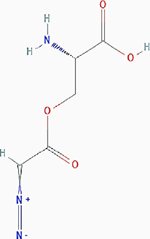
|
Irreversible inhibition | Nonselective. Glutamine utilizing enzymes, including glutamine amidotransferases, γ-glutamyltranspeptidase (GGT) | Inhibits GGT by covalently modifying the Thr391 residue at the active site. | PubChem CID: 460129 URL: https://pubchem.ncbi.nlm.nih.gov/compound/460129#section=2D-Structure [40,92] |
| l-γ-Glutamyl-p-nitroanilide (GPNA) |
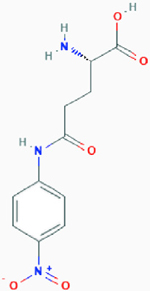
|
Competitive inhibition | Glutamine transporter SLC1A5, sodium-dependent and independent amino acid transporters | Proposed model: Inhibits SLC1A5 by binding to the binding site with high affinity (the ligand part binds to the lipophilic pocket of SLC1A5 and forms a hydrogen bond). | PubChem CID: 81732 URL: https://pubchem.ncbi.nlm.nih.gov/compound/81732#section=2D-Structure [47,48] |
| V-9302 |
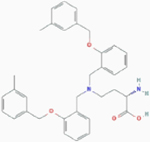
|
Competitive inhibition | Glutamine transporter SLC1A5 | In silicon model: V-9302 docks into the orthosteric binding site situated at the transmembrane region of SLC1A5. | PubChem CID: 127035871 URL: https://pubchem.ncbi.nlm.nih.gov/compound/127035871#section=2D-Structure [49] |
| Compound 968 |
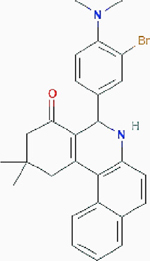
|
Allosteric inhibition | Glutaminase (GLS): kidney-type glutaminase: kidney glutaminase (KGA) and glutaminase C (GAC), and liver-type glutaminase (GLS2) | Inhibits glutaminases by binding to GAC monomers and preventing the formation of activated GAC tetramers. | PubChem CID: 3099980 URL: https://pubchem.ncbi.nlm.nih.gov/compound/3099980#section=2D-Structure [50–52] |
| bis-2-(5-phenylacetamido-1,2,4-thiadiazol-2-yl)ethyl sulfide (BPTES) |
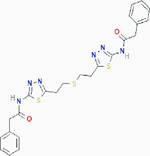
|
Allosteric inhibition | Selectively inhibits kidney-type glutaminase (GLS): kidney glutaminase (KGA) and glutaminase C (GAC) | Inhibits KGA and GAC by trapping their tetramers in a conformation that is nonfunctioning. | PubChem CID: 3372016 URL: https://pubchem.ncbi.nlm.nih.gov/compound/3372016#section=2D-Structure [93–95] |
| CB-839 (Telaglenastat) |
|
Allosteric inhibition | Selectively inhibits kidney-type glutaminase (GLS): kidney glutaminase (KGA) and glutaminase C (GAC) | Inhibits KGA and GAC by forming hydrogen bonds with cKGA (catalytic domain Ile221-Leu533 of KGA) tetramer. | PubChem CID: 71577426 URL: https://pubchem.ncbi.nlm.nih.gov/compound/71577426#section=2D-Structure [58,59] |
