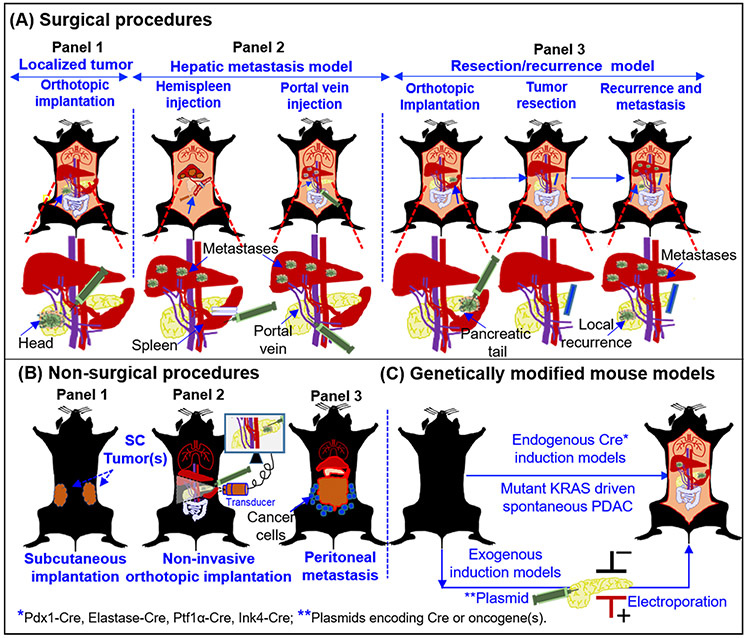
Figure 1. Major PDAC experimental mouse models employed for preclinical research.
Presentation of a surgical (A), non-surgical (B), and spontaneous models (C), for the experimental therapeutics of PDAC. (A) Panel 1: Orthotopic implantation of tumor source (cells/tumor derivatives) in the pancreas of the mouse. Lower panel depicts the approaches employed for implantation of cells and tumor growth in head of the pancreas. Panel 2: Hepatic metastases models are generated by hemispleen injection and portal vein injection. The lower panels indicate the injection site in the spleen (Left) and in the portal vein (Right). Details of the procedures are mentioned in the section 2.2.4. Panel 3: Resection model for the testing adjuvant and neoadjuvant therapies to evaluate their effect on local recurrence and metastasis. The lower panel depicts implantation of cells and tumor growth in the tail of the pancreas. Such models have allowed the R0 resections. Following resection mice can followed for local recurrence and metastasis .in the presence or absence of therapeutic modalities. (B) Non-surgical procedures: the SC implantation method (panel 1), non-invasive ultrasound guided (USG) injection model (panel 2), and peritoneal dissemination model (panel 3), are primarily the non-surgical methods to evaluate the therapeutics against primary tumor and peritoneal metastasis. (C) Genetically modified mouse models (GEMMs): Both endogenous and exogenous genetic modification can be performed to develop GEMMs of PDAC. Mouse models with mutant KRAS and/or TP53 genes driven by Cre-recombinases under the control of various cell-type specific promoters and described in the in the section 2.2.8. The spontaneous models recapitulate the pathological features of human PDAC with primary tumors and multiple metastases. Alternatively, exogenous induction of oncogenes can be achieved by the introduction of plasmids via electroporation or injectable viral particles with different Cre recombinases to induce the tumor growth in the pancreas.