FIG 3.
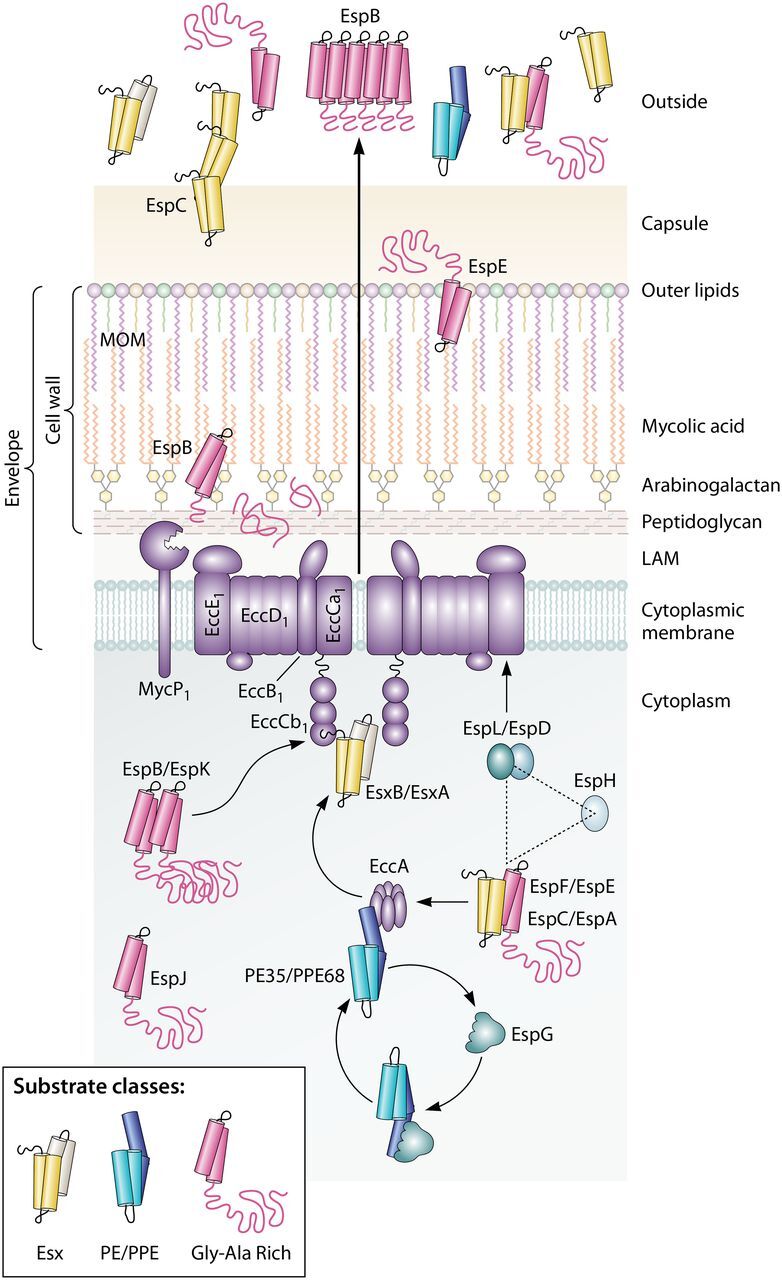
Conserved aspects of ESX-1 protein transport in M. tuberculosis and M. marinum. The schematic shows that the ESX-1 conserved components EccA to EccE interact with substrates and provide a channel across the cytoplasmic membrane. The three classes of conserved ESX-1 substrates are shown. The EccCb1 ATPase directly interacts with the EsxB and EspK substrates; the EccA ATPase interacts with the PPE68 and EspF substrates. Substrates are often targeted in pairs. The EspG chaperone interacts with PE/PPE substrate pairs and likely brings these substrates to the EccA component before being recycled. It is not known how substrates are transferred from the EccA component to the membrane complex. EspL and EccE interact with EspD. EspL and EspD are required for the stability of EspE, EspF, and EspH. Following transport through the membrane complex, ESX-1 substrates are transported across the envelope via an unknown mechanism. EspB is cleaved by the MycP1 protease. Substrates can be targeted to the MOM (mycolate outer membrane), to the capsule, and out of the cell. Some substrates, including EspC and EspB, form higher-order structures following secretion, which may indicate that they are part of the secretory machinery. LAM, lipoarabinomannan.
