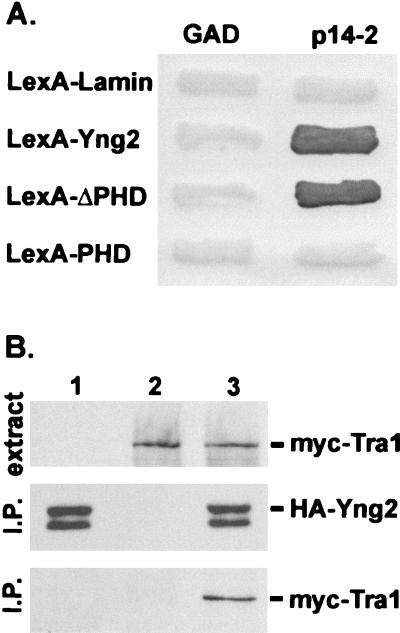
FIG. 5.
Yng2 is associated with Tra1. (A) The Gal activation domain (GAD) and a GAD fusion with Tra1 (residues 1678 to 1740) (p14-2) were tested for their ability to interact with LexA fusions with lamin, Yng2, the N-terminal domain (residues 1 to 222) of Yng2 (LexA-ΔPHD), or the C-terminal domain (residues 222 to 282) of Yng2 (LexA-PHD) by the yeast two-hybrid test (Materials and Methods). Each patch represents an independent transformant of the yeast two-hybrid tester strain L40 expressing the indicated proteins. Interaction between fusion proteins was assayed by their ability to induce expression of β-galactosidase by a filter assay (10). (B) Extracts from JC1 cells expressing HA-Yng2 (lane 1), myc-Tra1 (lane 2), or HA-Yng2 and myc-Tra1 (lane 3) were assayed for expression of myc-tagged proteins by Western blot analysis using anti-myc (monoclonal antibody 9E10) antibody (top panel). Ten milligrams of total protein from each extract was immunoprecipitated (I.P.) with anti-HA (monoclonal antibody 12CA5) antibody; half of the immunoprecipitate was probed with anti-HA antibody (middle panel), and half was probed with anti-myc antibody (bottom panel). Plasmids used to express proteins were pADHA-Yng2 and pUAD6 (lane 1), pAD4.H and pADmyc-Tra1 (lane 2), or pADHA-Yng2 and pADmyc-Tra1 (lane 3).