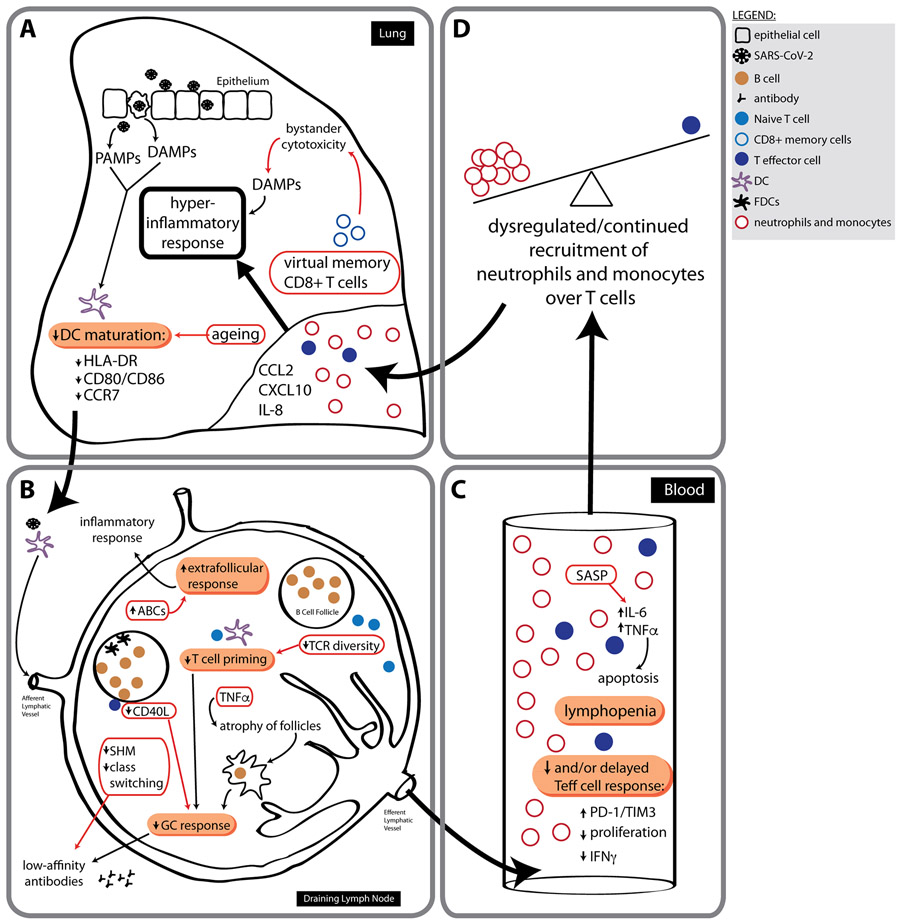
Figure 4. Ageing adaptive immune system responses during severe COVID-19.
a-d, It has been hypothesized that a delayed and/or weak adaptive immune response helps drive the dysregulated and continued recruitment of neutrophils and monocytes. These cells facilitate a non-specific and highly destructive hyperinflammatory response that contributes to the outcomes observed in patients with severe COVID-19. Failures of the adaptive system would begin at sites of infection (a), where DCs are initially activated by PAMPs and DAMPs in response to SARS-CoV-2. Patients with severe COVID-19 have DCs with a reduced maturation profile. (b) Ageing also negatively affects DC maturation, furthering the potential for improper priming of naive T cells in lymph nodes in older adults. There appears to be a loss of GC reactions and atrophy of the secondary lymphoid organs (SLOs) in fatal cases of COVID-19. These factors, combined with decreased CD40L expression on aged CD4+ T cells and reduced AID expression in aged B cells, could culminate in greatly impaired high-avidity, long-lasting antibody production that is dependent upon the success of GC reactions. Reductions in levels of GC reactions are also linked to extrafollicular and age-associated B cell (ABC) responses, which may potentiate ‘lupus-like autoimmune inflammation’. As we age, the naive TCR repertoire also decreases, making DC accessibility to SARS-CoV-2-specific naive T cells potentially even more challenging, delaying priming responses. (c,d) Lymphopenia is also strongly associated with severe COVID-19 outcomes. The aged microenvironment may contribute to this phenotype, as the SASP mediators IL-6 and TNFα can cause enhanced T cell apoptosis. Furthermore, the presence of virtual memory CD8+ T cells at sites of SARS-CoV-2 infection may contribute to a hyperinflammatory response through the action of bystander cytotoxicity, which occurs in a non-antigen-specific manner and can be highly destructive if left unregulated. AID, activation-induced cytidine deaminase; FDC, follicular dendritic cells; SHM, somatic hypermutation; Teff cell, effector T cell. Immune responses to the virus are indicated by black arrows, and consequences of aging are shown by red arrows.