Figure 6. Hercynine’s deuterium exchange with D2O when EanBY353F2Tyr variant is used as the catalyst and MetC using selenocystine as the selenium source.
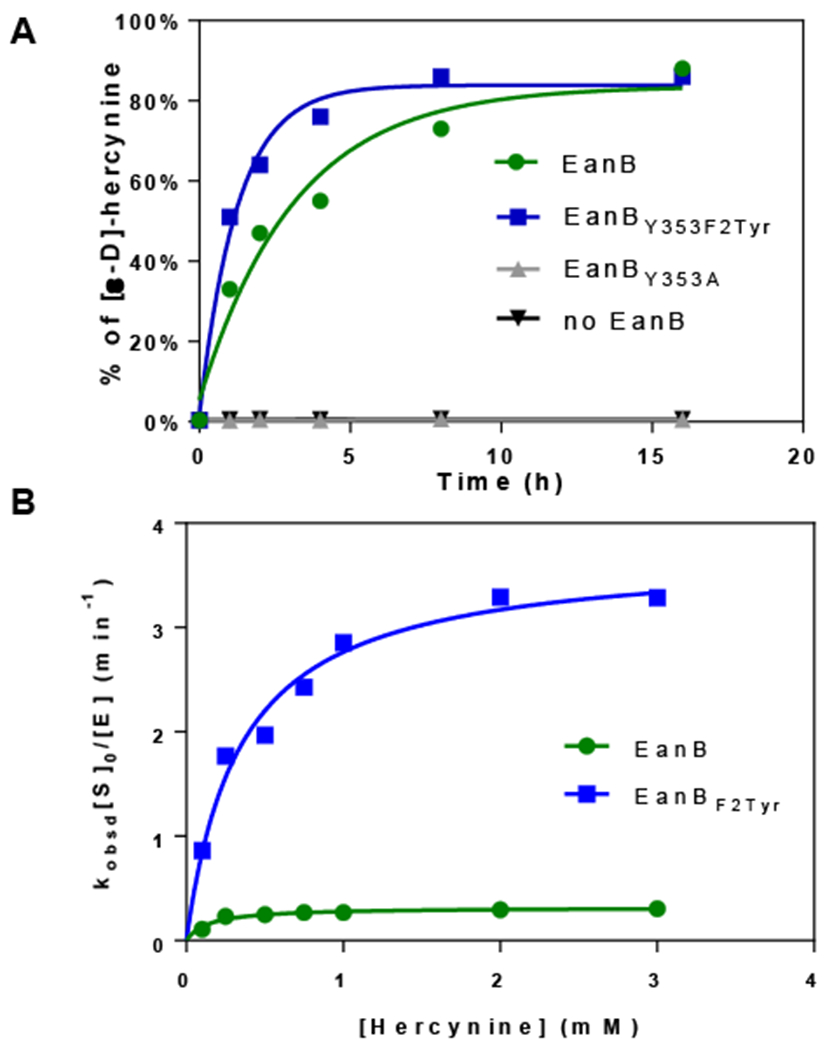
(A) Time course of the [ε-D]-hercynine formation under multiple turn-over conditions. The reaction mixture contained 50 μM of EanBY353A, EanBWT, or 12.5 μM EanBY353F2Tyr variant. (B) Hercynine’s deuterium exchange with D2O rate at various hercynine concentrations using EanB or EanBY353F2Tyr. The 1-ml reaction mixture contains 8 ~ 50 μM EanBWT (or 0.65 ~ 6.5 μM EanBY353F2Tyr), 100 ~ 3000 μM hercynine, selenocystine saturated solution (1 mg powder added), 0.5 μM MetC in 50 mM KPi buffer, with pD of 8.22. The Kobsd at different hercynine concentration is calculated based on the slope from the semilogarithmic plot of the reaction conversion vs time.
