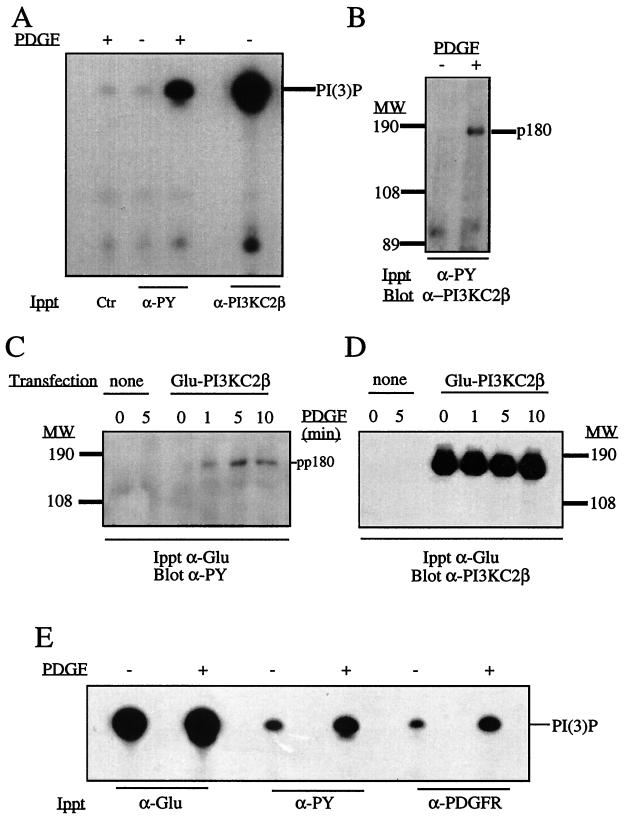
FIG. 6.
PI3K-C2β is present in antiphosphotyrosine antibody immunoprecipitates of PDGF-stimulated fibroblasts. (A and B) NIH 3T3 cells were stimulated with PDGF (10 nM), and lysates were immunoprecipitated with mouse immunoglobulin antibody (Ctr), antiphosphotyrosine antibody (α-PY), or anti–PI3K-C2β antiserum. The samples were assayed for PI3K in the presence of Ca2+, and the radioactive phosphoinositide products were separated by TLC (A). Antiphosphotyrosine antibody immunoprecipitates from quiescent or PDGF-stimulated cultures were also fractionated by SDS-PAGE and Western blotted with anti–PI3K-C2β antiserum (B). (C and D) Cultures of cells stably expressing epitope-tagged PI3K-C2β were stimulated with PDGF for various times as indicated, and their lysates were immunoprecipitated with anti-Glu tag monoclonal antibody. After SDS-PAGE, immunoprecipitated proteins were Western blotted with antiphosphotyrosine antibody (C), stripped, and reprobed with anti–PI3K-C2β antiserum (D). (E) Lysates from NIH 3T3 cells stimulated with PDGF were also immunoprecipitated with anti-Glu tag antibody, antiphosphotyrosine antibody, or anti-PDGFR antibody. The resultant immune complexes were analyzed for phospholipid kinase activity in the presence of Ca2+. Radiolabeled phosphoinositide products were separated by TLC. MW, molecular weight.