ABSTRACT
The signaling network formed by external environmental signals and endogenous hormone signals is an important basis for the adaptive growth of plants. We recently identified a UDP-glucosyltransferase gene, UGT76F1, which controls the glucosylation of auxin precursor IPyA and mediates light-temperature signaling to regulate auxin-dependent hypocotyl elongation in Arabidopsis. However, it is unclear whether UGT76F1 is involved in the adaptive growth of other tissues and whether it is related to the signaling of other hormones besides auxin. Here we investigated the petiole elongation of UGT76F1 overexpression lines and knockout mutant lines, and also studied the effects of UGT76F1 on BR signaling. Experimental results indicated that UGT76F1 is involved in the PIF4-mediated petiole growth under high temperature and that UGT76F1 is also related to the BR signaling in controlling hypocotyl growth. These results suggest that UGT76F1 may have a wider significance in the plant adaptations to surrounding environments.
KEYWORDS: Glycosyltransferase, petiole elongation, BR signaling, growth regulation
In the long evolutionary process of plants, the surrounding environmental factors provide motivation for the adaptive growth of plants under natural selection. When sensing external environments such as changing light signals and temperature signals, plants can make alterations in the growth and development in order to adapt to these changes. For example, exposure to high temperature will result in dramatic changes in Arabidopsis growths, including rapid extension of plant axes, leaf hyponasty, and early flowering.1,2 For optimal growth and development, the perception and processing of surrounding environmental information are crucial to plant performance. Besides external environment, plant hormones play another key regulatory role in this process. It has been revealed that the coordinated regulation between ambient temperature and hormones, especially auxin, is important in the temperature-promoted hypocotyl elongation.1,3 In addition, Phytochrome-interacting factor 4 (PIF4) is believed to be a cruel component of environmental signaling and integrate multiple hormone signals during plant development.4–8 Thus, the signal network formed by the transmission of external environmental signals through endogenous hormone signals is the basis for the adaptive growth of plants.
We recently identified a UDP-glucosyltransferase gene, UGT76F1, which controls the glucosylation of auxin precursor IPyA and mediates light-temperature signaling to regulate auxin-dependent hypocotyl elongation in Arabidopsis.9 However, it is unclear whether UGT76F1 is involved in the adaptive growth of other tissues and whether it is related to the signalings of other hormones besides auxin. To answer these questions, we investigated the petiole elongation under 22°C and 28°C using UGT76F1 overexpression lines and knockout mutant lines. In addition, we also studied the effects of UGT76F1 on BR signaling in controlling hypocotyl elongation.
After growing under 22°C for 4 weeks, ugt76f1 knockout mutant (KO) lines displayed longer petioles whereas UGT76F1 overexpression (OE) lines exhibited shorter petioles than WT (Figure 1a,b). Petiole extension of UGT76F1 transgenic seedlings was further investigated at 28°C after 2 weeks grown at 22°C (Figure 1c). The petioles of ugt76f1 mutant lines were markedly increased than that of WT at 28°C (Figure 1d,e), suggesting ugt76f1 mutants were more sensitive to elevated temperature. However, the petiole elongation ratio of 28°C/22°C in UGT76F1OE lines was decreased than that of WT (Figure 1e). It is known that PIF4 acts as a negative regulator of UGT76F1 transcription in high-temperature-induced hypocotyl elongation.9 Here, we thus genetically analyzed the role of UGT76F1 in PIF4-mediated and high-temperature-induced petiole elongation by comparing petiole length between pif4 and pif4ugt76f1ko as well as between 35 S:PIF4 and 35 S:PIF4UGT76F1OE lines. Intriguingly, while petiole growth response to high temperature was abolished in pif4 mutants, the pif4ugt76f1ko double mutants complemented the petiole growth phenotypes of pif4 (Figure 2a,b). Conversely, the 35 S:PIF4UGT76F1OE developed much shorter petioles than 35 S:PIF4 under high temperature (Figure 2c,d). These observations suggest that UGT76F1 genetically acts down-stream of PIF4 in high-temperature-induced petiole growth.
Figure 1.
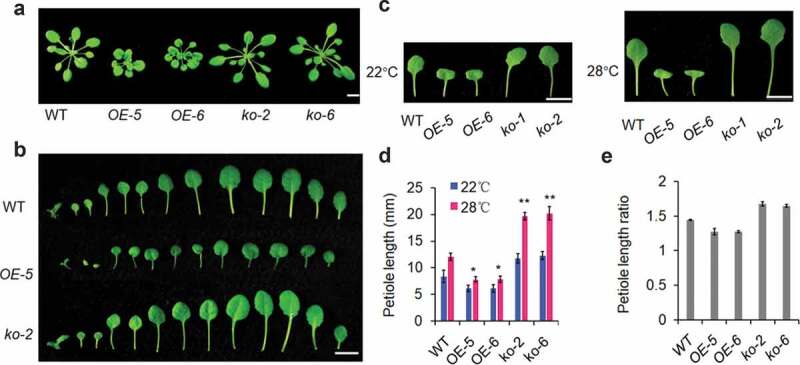
Temperature-mediated petiole growth in UGT76F1 overexpression lines (OE) and mutant lines (KO) under 22°C and 28°C.
(a-b) Petiole lengths of WT, UGT76F1OE lines, and ugt76f1 mutants grown at 22°C. Four-week plants were photographed (Scale bar = 1 cm). (c) Petiole lengths of WT, UGT76F1OE lines and ugt76f1 mutants grown at 22°C for 4 weeks or at 22°C for 2 weeks and then transferred to 28°C for another 2 weeks (Scale bar = 1 cm).(d) Petiole lengths of seedlings shown in (c). Data are means ± SD, n = 30. Significant difference was compared to respective value at 22°C. Student’s t-test was performed (*P < 0.05, **P < 0.01).(e) Petiole length ratios (28°C/22°C) of the quantified petiole length in (d). Experiments were conducted for three biological replicates, yielding similar results.
Figure 2.
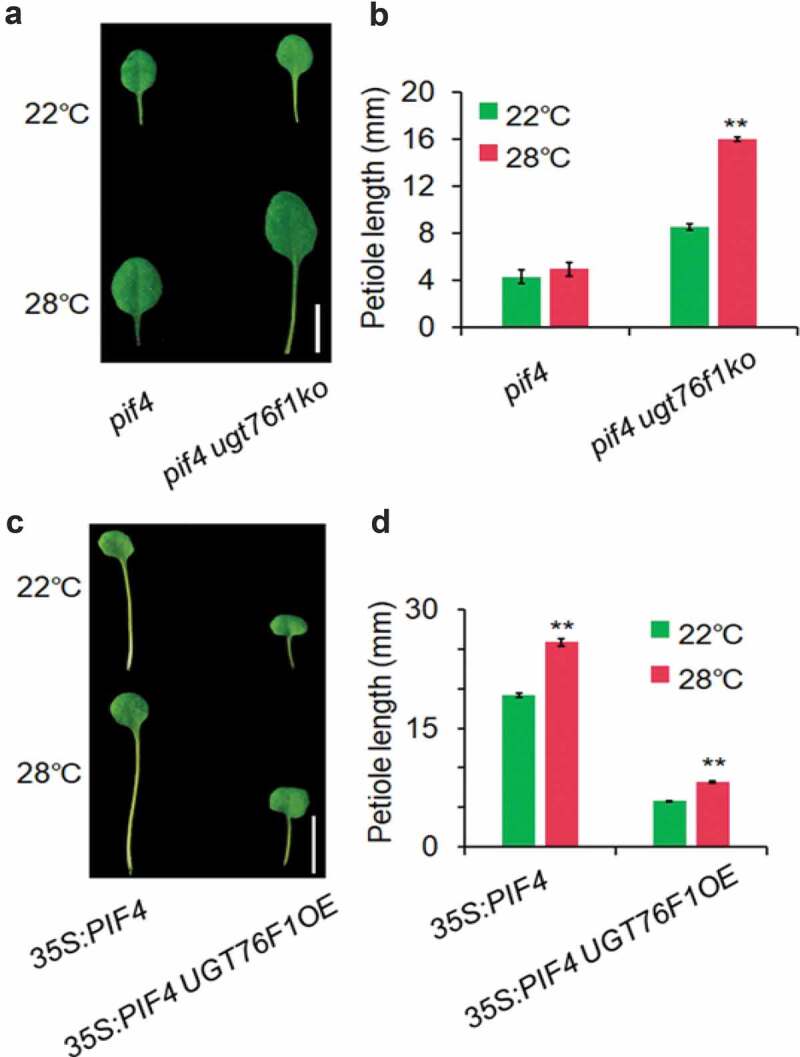
UGT76F1 is involved in PIF4-mediated and temperature-promoted petiole elongation.
(a) Phenotypes of pif4 and pif4ugt76f1ko seedlings grown at 22°C for 4 weeks or at 22°C for 2 weeks and then transferred to 28°C for another 2 weeks (Scale bar = 1 cm). (b) Petiole lengths of seedlings shown in (a). Data are means ± SD, n = 30. Significant difference was compared to respective value at 22°C. Student’s t-test was performed (**P < 0.01).(c) Phenotypes of 35 S:PIF4 and 35 S:PIF4UGT76F1OE seedlings grown at 22°C for 4 weeks or at 22°C for 2 weeks and then transferred to 28°C for another 2 weeks (Scale bar = 1 cm).(d) Petiole lengths of seedlings shown in (c). Data are means ± SD, n = 30. Significant difference was compared to respective value at 22°C. Student’s t-test was performed (**P < 0.01).
From our recent research, we know that UGT76F1 regulates auxin-dependent hypocotyl elongation.9 It has also been revealed in several studies that auxin, BR, and PIF4 interplay to control the elongation of hypocotyl cells.10–12 Here, we determined whether UGT76F1 is involved in BR signaling in hypocotyl elongation. UGT76F1OE lines and ugt76f1 mutants were exposed to various concentrations of exogenous brassinolide (BL, the most active form of BR) or BR biosynthesis inhibitor propiconazole (PPZ) at 28°C for 4 d. We found that overexpression of UGT76F1 reduced the sensitivity of seedlings to BR treatment in hypocotyl elongation compared to WT (Figure 3a,c). Likewise, the knockout of UGT76F1 reduced the sensitivity of seedlings to BR inhibitor in hypocotyl elongation (Figure 3b,d). These results suggest that UGT76F1 is also related to the BR signaling in controlling plant growth.
Figure 3.
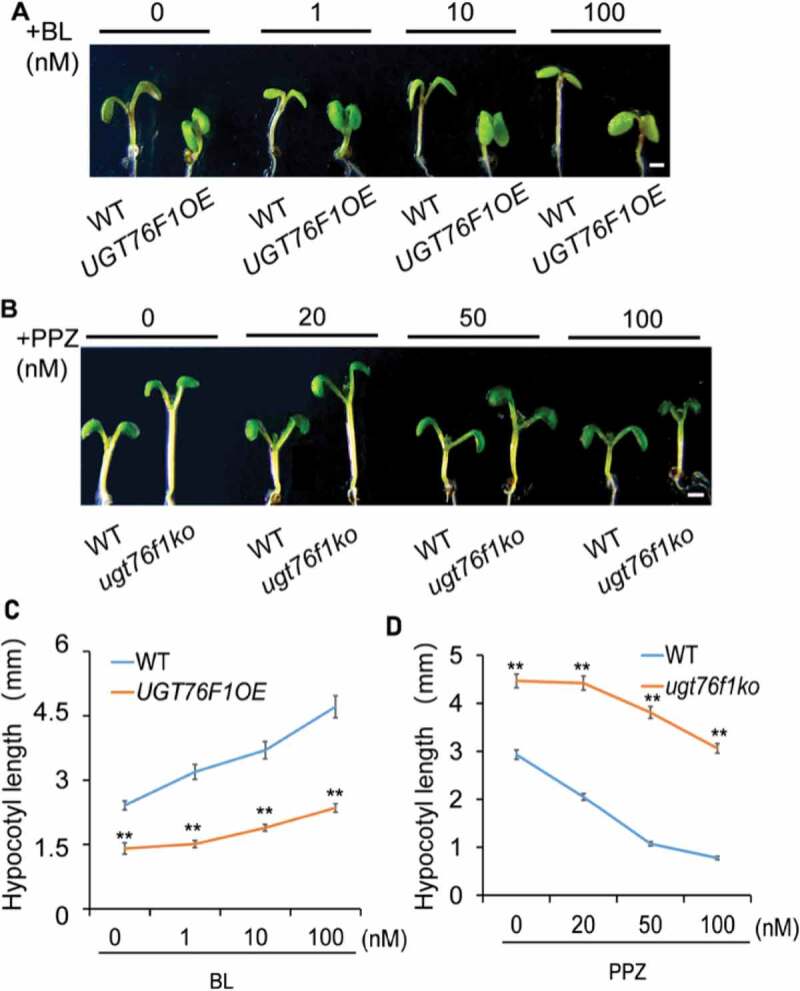
UGT76F1 affects BR-mediated hypocotyl elongation.
(a and c) The UGT76F1 overexpression lines reduced the sensitivity to BL. Seedlings were grown on media containing various concentrations of BL at 28°C for 4 d. Representative seedlings grown on either mock (0 nM BL) or BL (1–100 nM BL) media are shown in (a). (b and d) The ugt76f1 mutant lines reduced the sensitivity to PPZ. Seedlings were grown either on mock (0 nM PPZ) or PPZ (20–100 nM PPZ) at 28°C for 4 d. Data are means ± SD, n = 30. Significant difference was compared to respective WT. Student’s t-test was performed (**P < 0.01).
Previously, it has been shown that BR-regulated BZR1, auxin-regulated ARF6, and light/temperature-regulated PIF4, interact with each other and cooperatively regulate hypocotyl cell elongation.11 Based on the observation in this study and our recent research, 9 we propose that UGT76F1 functions in the adaptive growth of plants to external environments likely downstream of the network regulation including auxin, BR, and PIF4. Since UGT76F1 is a glycosyltransferase toward auxin precursor IPyA, the glycosylation toward the metabolites of other plant hormones may also play important roles in the plant adaptations to surrounding environments.
Acknowledgments
We thank Prof. Zhaojun Ding (Shandong University) for providing the pif4 and 35S:PIF4 seeds. This research is supported by grants from National Natural Science Foundation of China (No.31770313, No. 91217301 and No. 31970290 to B.K.H).
Funding Statement
This work was supported by the National Natural Science Foundation of China [No.31770313, No. 91217301 and No. 31970290].
References
- 1.Gray WM, Ostin A, Sandberg G, Romano CP, Estelle M.. High temperature promotes auxin-mediated hypocotyl elongation. Arabidopsis Proc Natl Acad Sci USA. 1998;95:1–4. doi: 10.1073/pnas.95.12.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balasubramanian S, Sureshkumar S, Lempe J, Weigel D.. Potent induction of Arabidopsis thaliana flowering by elevated growth temperature. PLoS Gen. 2006;2:106. doi: 10.1371/journal.pgen.0020106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stavang JA, Gallego‐Bartolomé J, Gómez MD, Yoshida S, Asami T, Olsen JE, García‐Martínez JL, Alabadí D, Blázquez MA. Hormonal regulation of temperature-induced growth. Arabidopsis Plant J. 2009;60:589–601. [DOI] [PubMed] [Google Scholar]
- 4.Lucyshyn D, Wigge PA. Plant development: PIF4 integrates diverse environmental signals. Curr Biol. 2009;19:R265–266. doi: 10.1016/j.cub.2009.01.051. [DOI] [PubMed] [Google Scholar]
- 5.Leivar P, Quail PH. PIFs: pivotal components in a cellular signaling hub. Trends Plant Sci. 2011;16:19–28. doi: 10.1016/j.tplants.2010.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Proveniers MC, van Zanten M. High temperature acclimation through PIF4 signaling. Trends Plant Sci. 2013;18:59–64. doi: 10.1016/j.tplants.2012.09.002. [DOI] [PubMed] [Google Scholar]
- 7.Quint M, Delker C, Franklin KA, Wigge PA, Halliday KJ, Zanten M. Molecular and genetic control of plantthermomorphogenesis. Nat Plants. 2016;2:15190. doi: 10.1038/nplants.2015.190. [DOI] [PubMed] [Google Scholar]
- 8.Huai J, Zhang X, Li J, Ma T, Zha P, Jing Y, Lin R. SEUSS and PIF4 coordinately regulate light and temperature signaling pathways to control plant growth. Mol Plant. 2018;11:928–942. doi: 10.1016/j.molp.2018.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Chen L, Huang XX, Zhao SM, Xiao DW, Xiao LT, Tong JH, Wang WS, Li YJ, Ding Z, Hou BK. IPyAglucosylation mediates light and temperature signaling to regulate auxin-dependent hypocotyl elongation in Arabidopsis. Proc.Natl. Acad. Sci. USA. 2020;117:6910–6917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hardtke CS. Transcriptional auxin-brassinosteroid crosstalk: who’s talking? Bioessays. 2007;29:1115–1123. doi: 10.1002/bies.20653. [DOI] [PubMed] [Google Scholar]
- 11.Oh E, Zhu JY, Bai MY, Arenhart RA, Sun Y, Wang ZY. Cell elongation is regulated through a central circuit of interacting transcription factors in the Arabidopsis hypocotyl. Elife. 2014;3:e03031. doi: 10.7554/eLife.03031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oh E, Zhu JY, Wang ZY. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat Cell Biol. 2012;14:802–809. doi: 10.1038/ncb2545. [DOI] [PMC free article] [PubMed] [Google Scholar]


