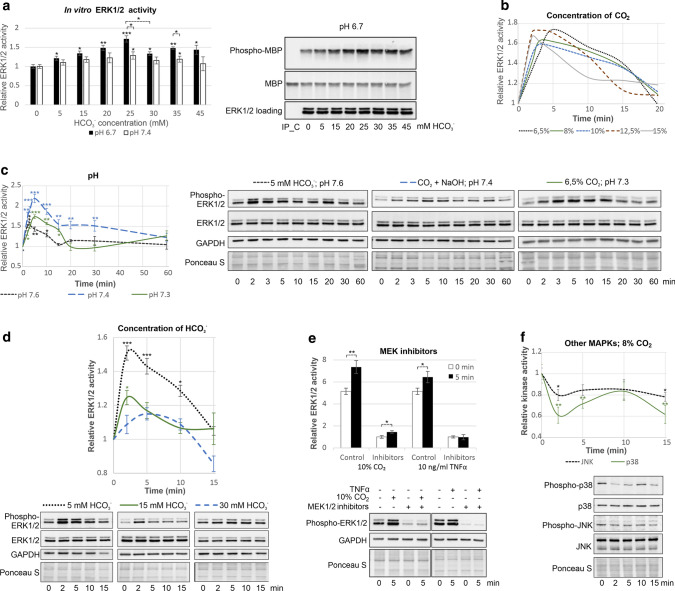
Fig. 1.
MAPKs are regulated by CO2. a CO2 directly regulates ERK1/2 activity in vitro. ERK1/2 immunoprecipitated from ECs was dephosphorylated and subjected to an in vitro kinase activity assay in the presence of the indicated concentration of HCO3−. Phosphorylation of MBP, which was used as an ERK1/2 substrate for in vitro phosphorylation reactions, was measured by immunoblotting with an anti-phospho-MBP antibody. The levels of ERK1/2 were determined using immunoblotting with an anti-ERK1/2 antibody, and MBP was visualised by Ponceau S staining. An immunoprecipitation control (IP_C), i.e. Dynabeads Protein G incubated with cell lysates without the anti-ERK1/2 antibody, was used to show the specificity of the assay. b In ECs, ERK1/2 were transiently activated by CO2 at a wide range of concentrations. Graphs for the individual CO2 concentrations with bars representing the SD are presented in Fig. S1a. c CO2 promotes ERK1/2 activation regardless of pH. Time courses of ERK1/2 activation at pH 7.4 (CO2 neutralised by NaOH) and at a lower (6.5% CO2) or higher (NaHCO3) pH are shown. d Time courses of HCO3−-induced ERK1/2 activation in ECs. e ERK1/2 were activated by CO2 independently of upstream MEK1/2 in ECs, as MEK1/2 inhibitors (2.5 µM U0126 and 25 µM PD98059) did not abolish the CO2-induced ERK1/2 activation but did prevent ERK1/2 activation by 10 ng/ml TNFα. In b-e, ERK1/2 activity was measured by immunoblotting with an anti-phospho-ERK1/2 antibody. Protein loading was assessed by both Ponceau S staining and immunoblotting with anti-GAPDH and anti-ERK1/2 antibodies. f JNK and p38 were inactivated by CO2 in ECs. JNK and p38 activity was determined by immunoblotting with anti-phospho-JNK and anti-phospho-p38 antibodies, respectively. The results of three independent experiments are presented