Figure 4. Unique active sites facilitate SidJ-mediated glutamylation of the SidE effectors.
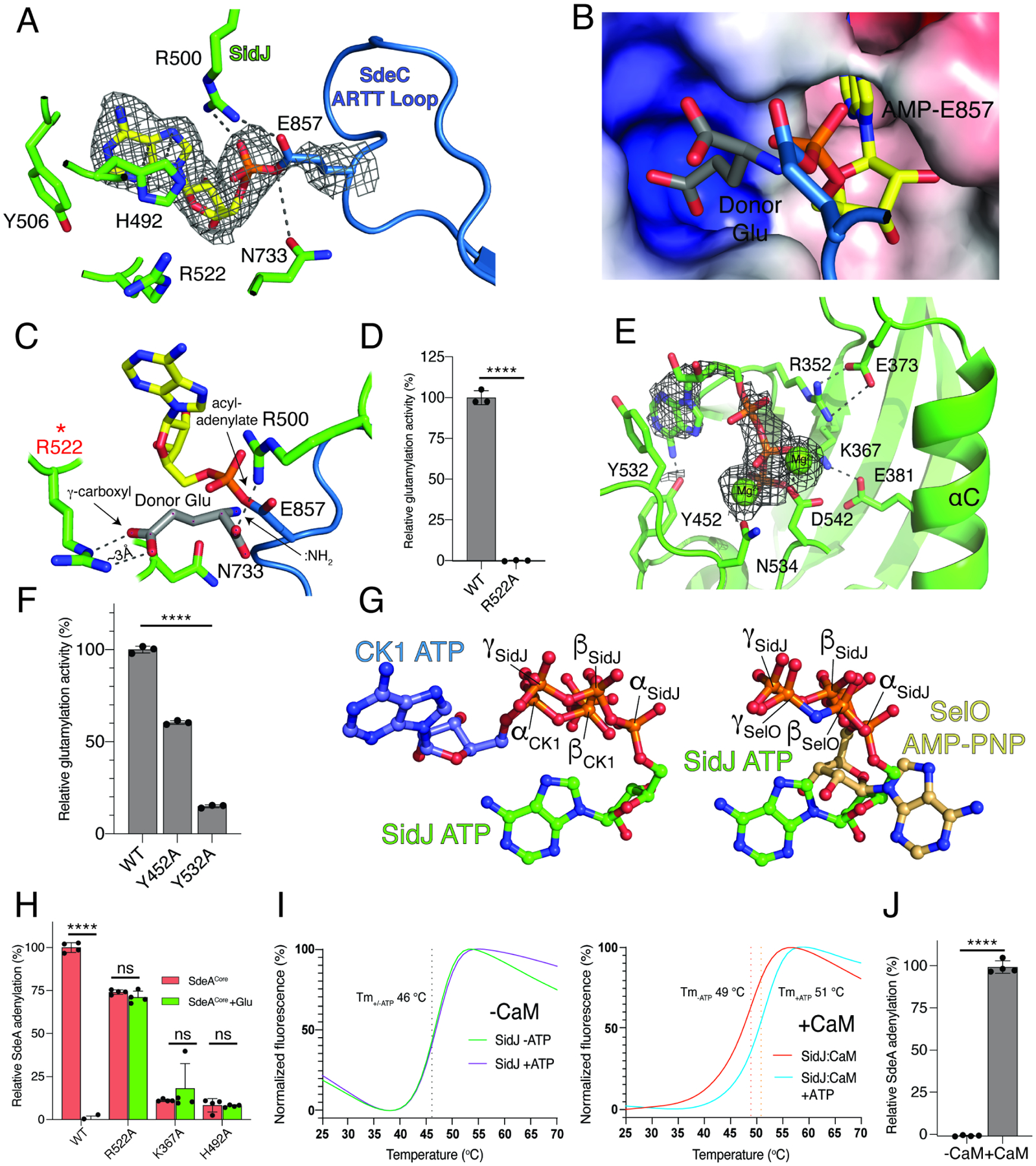
(A) Details of the migrated nucleotide binding pocket of SidJ (green) and the ARTT loop of SdeCCore (blue) showing the interactions (dashed lines) involved in binding the acyl-adenylate reaction intermediate. The AMP is shown as sticks and the Coulomb potential map is shown in mesh. Note the formation of a covalent bond between AMP and the SdeC active site Glu857.
(B, C) Electrostatic surface representation (B) and molecular interactions (C) of the migrated nucleotide binding pocket of SidJ bound to SdeCCore depicting a positively charged cleft adjacent to the acyl-adenylate reaction intermediate. A donor Glu was manually placed inside of the pocket, with its γ-carboxyl group interacting with Arg522 of SidJ. The donor Glu :NH2 group is in position to attack the acyl-adenylate. Electrostatic surface is contoured at 7kT.
(D) Glutamylation activity of SidJ and the Arg522 mutant using SdeACore as substrate. Reaction products were analyzed as in Figure 2C, n=3.
(E) Details of the kinase-like active site of SidJ (green) depicting the molecular interactions (dashed lines) that facilitate ATP/Mg2+ binding. The ATP is in sticks, the Coulomb potential map is shown in mesh and the Mg2+ ions are shown as spheres.
(F) Glutamylation activity of SidJ and kinase-like active site mutants using SdeACore as a substrate. Reaction products were analyzed as in Figure 2C, n=3. Note that the other residues have been mutated and analyzed for glutamylation in our previous work (Black et al., 2019).
(G) Ball-and-stick representation of superimposed nucleotides from SidJ and protein kinase CK1 (left) and SidJ and SelO (right) as a result of superposition of the kinase active sites. The α, β, and γ-phosphates of SidJ, SelO and CK1 are highlighted. Note that the nucleotide orientation of SidJ is similar to SelO, which transfers AMP to proteins (Sreelatha et al., 2018).
(H) Quantification of acyl-adenylate formation following reactions with SidJ59–851 (WT and mutants), SdeACore and [α-32P]ATP. SdeACore E860Q was used to calculate the baseline signal in TCA precipitates. The reactions were terminated by the addition of TCA and the SdeA acyl-adenylate was detected by scintillation counting of the acid-insoluble material (red bars), n=4. Glu was also added to the reactions (green bars), which displaces the 32P-AMP from SdeA into a TCA soluble fraction.
(I) Differential scanning fluorimetry (DSF) depicting the thermal stability profiles of SidJ (left) and SidJ:CaM (right) in the presence or absence of ATP/Mg2+. Protein denaturation was followed by monitoring the fluorescence of SYPRO Orange dye, which binds hydrophobic regions on proteins. The Tm values are shown in the insets. Graphs show a mean of separately normalized triplicates.
(J) Quantification of acyl-adenylate formation following reactions with SidJ59–851, SdeACore, [α-32P]ATP in the presence (+) or absence (−) of CaM. SdeACore E860Q was used to calculate the baseline signal in TCA precipitates. Reaction products were analyzed as in Figure 4H, n=3.
Data in graphs shown as mean ± SD, ****P <0.0001, ns >0.15.
