Figure 7. SidJ and SdjA differentially regulate the SidE-Ub ligases during Legionella infection.
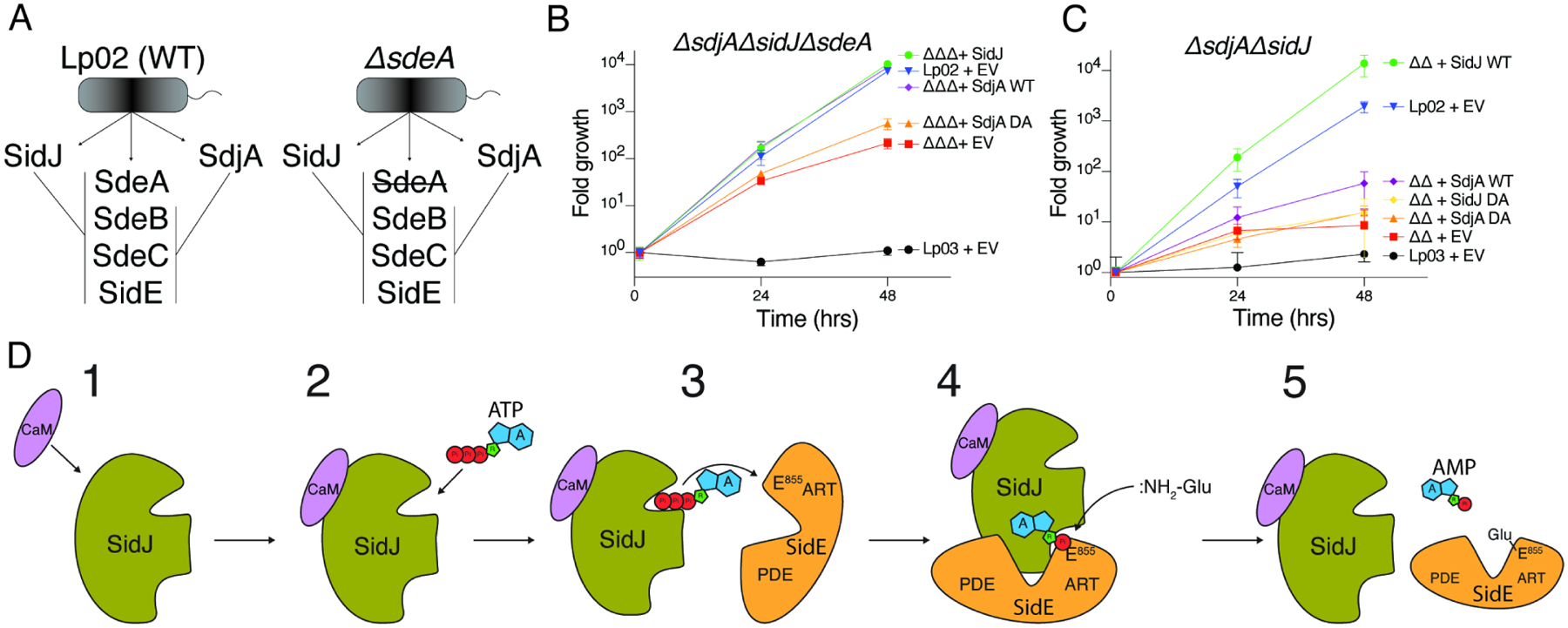
(A) Schematic representation of SidJ/SdjA genetic interaction during Legionella infection. In a wild-type L. pneumophila (Lp02) strain, SdjA is unable to complement SidJ because it is inactive against SdeA (left). In a ΔsdeA background, SdjA is functionally redundant with SidJ (right).
(B, C) Replication of L. pneumophila strains in A. castellanii. Infected amoeba cells were lysed at the indicated timepoints and bacterial replication was quantified by plating serial dilutions of lysates. Results are representative of two independent experiments; error bars denote SD from 1 experiment performed in triplicate. ΔsdjAΔsidJΔsdeA (ΔΔΔ), ΔsidJΔsdeA (ΔΔ), EV; empty vector, DA denotes SidJD542A or SdjAD480A, Lp03; ΔdotA (T4SS deficient). Error bars represent standard deviation.
(D) Model of SidJ-catalyzed glutamylation of the SidE effectors. (1) SidJ is translocated into host cells and binds CaM. (2) CaM binding renders the kinase-like active site competent to bind ATP/Mg2+ and (3) adenylate the active site Glu in the SidE effectors. (4) Adenylated SidE binds the migrated nucleotide binding pocket of SidJ, forming a stable reaction intermediate. Free Glu binds to a positively charged cleft in the migrated nucleotide binding pocket of SidJ which positions the NH2 group for nucleophilic attack of the acyl-adenylate, (5) releasing AMP and forming an isopeptide bond between Glu and SidE.
