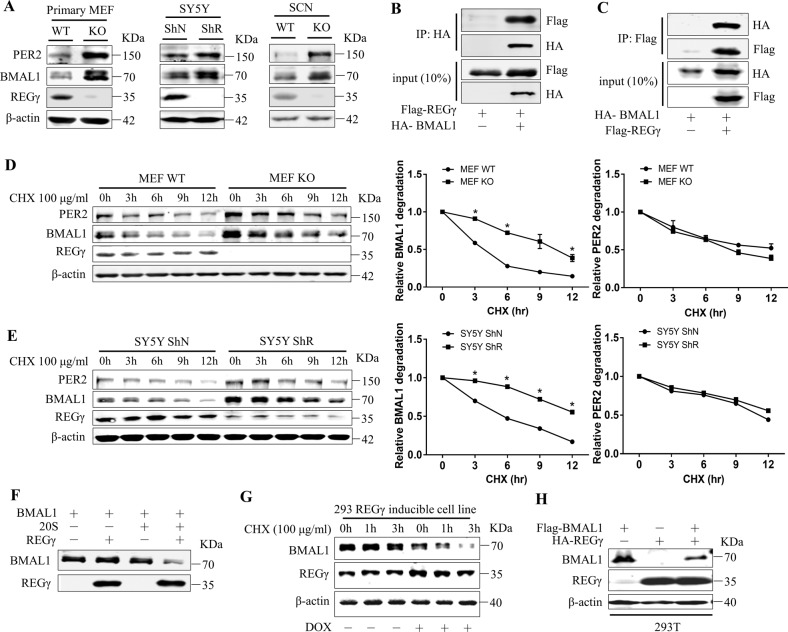
Fig. 4. REGγ directly interacts with BMAL1 and promotes its degradation.
A Expression of REGγ, BMAL1, and PER2 in MEF WT/KO, SY5Y ShN/ShR cells, and REGγ WT/KO mouse SCN. B Interaction between REGγ and BMAL1, determined by co-immunoprecipitation and western blot analysis following transient transfection of 2 μg of HA-BMAL1 and 2 μg of Flag-REGγ into 293T cells. C Reciprocal interaction between REGγ and BMAL1 was analyzed by transient transfection of 2 μg of Flag-REGγ and 2 μg of HA-BMAL1 into 293T cells. D, E Stability of endogenous BMAL1 in MEFs and in SY5Y cells was analyzed in the presence of CHX (100 μg/ml) for the indicated time points followed by western blot analysis. F In vitro proteolytic analysis of REGγ-mediated degradation of BMAL1. Purified REGγ, 20S proteasome, and in vitro-translated BMAL1 were incubated at 30 °C for 3 h as indicated. G Expression of REGγ was induced by doxycycline (1 μg/ml DOX for 48 h) in an engineered 293 cell line followed by western blot analysis of BMAL1 stability in the presence of cycloheximide as indicated. H 293T cells were transfected with Flag-BMAL1 and HA-REGγ followed by western blot analysis for REGγ and BMAL1 correlation. The quantitative results of BMAL1 stability in D, E were plotted to indicate dynamic changes. Values represent mean ± SD. *p < 0.05; t test, WT vs. KO; ShN vs. ShR.