Abstract
Background:
Intraoperative balloon electronic brachytherapy (IBEB) may provide potential benefit for local control of recurrent cerebral glioblastomas (GBMs).
Methods:
This is a preliminary report of an open-label, prospective, comparative cohort study conducted in two neurosurgical centers with ongoing follow-up. At recurrence, patients at one center (n = 15) underwent reresection with IBEB while, at the second center (n = 15), control subjects underwent re-resection with various accepted second-line adjuvant chemoradiotherapy options. A comparative analysis of overall survival (OS) and local progression-free survival (LPFS) following re-resection was performed. Exploratory subgroup analysis based on postoperative residual contrast-enhanced volume status was also done.
Results:
In the IBEB group, median LPFS after re-resection was significantly longer than in the control group (8.0 vs. 6.0 months; log rank χ2 = 4.93, P = 0.026, P < 0.05). In addition, the median OS after second resection in the IBEB group was also significantly longer than in the control group (11.0 vs. 8.0 months; log rank χ2 = 4.23, P = 0.04, P < 0.05).
Conclusion:
These hypothesis-generating results from a small cohort of subjects suggest putative clinical benefit in OS and LPFS associated with maximal safe re-resection of recurrent GBM with IBEB versus re-resection and standard adjuvant therapy, a hypothesis that deserves further testing in an appropriately powered clinical trial.
Keywords: Brachytherapy, Glioblastoma, Glioma resection, Intraoperative radiation therapy, Radiation therapy

INTRODUCTION
Glioblastoma (GBM) is the most frequently occurring malignant intracerebral neoplasm in adults. It is characterized by significant infiltrative growth and an aggressive clinical course.[14,27] Resection of those tumors is not curative and requires further adjuvant treatment targeting the macro- and microscopic residual tumor.[34] The therapeutic standard for newly diagnosed GBMs involves maximal safe resection with adjuvant external beam radiotherapy (EBRT; 60 Gy in 30 fractions of 2 Gy each), coupled with daily adjuvant temozolomide, followed by 6 to 12 cycles of maintenance chemotherapy. Recently, the incorporation of Tumor Treating Fields (TTField) therapy has been shown to further prolong overall survival (OS).[41] This has significantly improved the outcome of patients with GBM, especially those with O6-methylguanine-DNA methyltransferase (MGMT) gene[40] methylation. Local recurrence occurs most frequently within 2 cm of the resection cavity.[32] There is no general agreement on standard of care for patients with recurrent GBM, since no therapy has categorically been demonstrated to improve OS, although progression-free survival reresection[20] can prolong BCNU wafers,[13,48] bevacizumab,[3,16] and EBRT.[18] TTField therapy is approved for use in recurrent malignant glioma, but also fails to prolong OS.[41] Active investigational efforts are obviously required to develop more efficacious therapeutic approaches.[46]
Because of the obvious difficulty in conducting a randomized trial of gross total versus lesser resection in GBM, level 1 data supporting the value of gross total tumor resection for prolonging OS are rather spare,[8,39,47] but large retrospective cohort studies[25,29] have been used to impute the therapeutic value of more complete resection in the up-front setting. In the context of re-resection for recurrent GBM, level 1 evidence continues to remain even sparser. In clinical practice, maximal safe resection has largely been adopted as a component of therapy at recurrence because of the clinical benefit of relieving mass effect symptoms and possibly enhancing the efficacy of adjuvant therapy, since chemoradiotherapy, as well as the body’s own immune responses, is relatively more effective at controlling smaller tumor volumes.[7,17,24]
Level 1 data strongly support the role of radiation therapy in the management of newly diagnosed malignant gliomas; however, the use of EBRT in recurrent malignant glioma is often limited by the relatively high risk of radiation-related complications. The value of re-irradiation on OS, progression-free survival, and quality of life is still not well documented and needs to be investigated in future prospective trials.[2] A recent randomized trial that added EBRT to systemic bevacizumab did not prolonge median OS versus bevacizumab alone but did improve progression-free survival.[45] Other non-randomized EBRT approaches have utilized various fractionation schema,[10] alterations in the dose rate[1] or approaches combining’s EBRT to bevacizumab.[5]
Attempts to use various intraoperative radiation therapy (IORT) techniques in patients with malignant gliomas have been undertaken for several decades. Several studies have shown improved survival rates in subjects treated with IORT compared with retrospective controls; however, these studies were performed in small groups of subjects, and exhibits significant limitations.[9] Interstitial brachytherapy[37] was used in the 1980s and 1990s. An inflatable balloon catheter containing liquid 125I has been developed for treating recurrent malignant glioma.[44] Chan et al. reported prolongation of OS in subjects with recurrent GBM compared to historical controls. These subjects (n = 24) were treated perioperatively with an 125I radioactive fluid requiring the balloon to be left in situ for several days.[4,12]
Intraoperative balloon electronic brachytherapy (IBEB) is a new method that has been successfully applied in oncological practice over the past few years to treat breast cancer as well as other neoplasms.[21] IBEB offers several hypothetical advantages, such as rapid procedure implementation, manageable workflow, an opportunity for thorough preoperative radiotherapy planning, and intraoperative monitoring. A single, short session can permit delivery of it prescribed single focal dose equivalent to multiple sessions of fractionated external irradiation, while also minimizes radiation dose to the neighboring healthy tissue.[22] The objective of the following pilot study was to assess the clinical benefit of combining repeat resection of recurrent GBM with IBEB.
MATERIALS AND METHODS
Study subjects
This prospective cohort study was performed at two tertiary referral neurosurgical centers in the Russian Federation. The study enrolled in total 30 ≥18 years old patients with recurrent GBM (imaging-defined, using brain magnetic resonance imaging [MRI] with contrast enhancement and MR-perfusion) with Karnofsky Performance Status (KPS) ≥60%. Ineligibility criteria included contraindications to general anesthesia, decompensated chronic illness, acute infectious and non-infectious inflammatory processes, inability to undergo MRI with contrast enhancement and/or positron emission tomography-computed tomography (PETCT) with amino acid tracers, pregnancy, or breastfeeding. The final decision on subject inclusion was made by a multidisciplinary team with the mandatory participation of a neurosurgeon, a radiologist, a radiation oncologist, and a medical oncologist.
IBEB device
The electronic brachytherapy device used in this study is a miniaturized X-ray source that applies electronic brachytherapy (Xoft® Axxent® Electronic Brachytherapy (eBx®) System; Xoft®, a subsidiary of iCAD, Inc., San Jose, CA USA). The device enables highly focused therapeutic radiation to the target tissue with very rapid dose fall-off, which spares surrounding tissue. The X-ray source is complemented by a range of balloon applicators to be filled with varying volumes of saline to optimally fit the contour of the surgical cavity. This provides a well-defined geometry for the miniaturized X-ray source and allows the delivery of a more conformal radiation dose.
The Xoft System [Figure 1] is FDA-approved, CE marked and is licensed in a growing number of countries for the treatment of cancer.[26,28,38]
Figure 1:
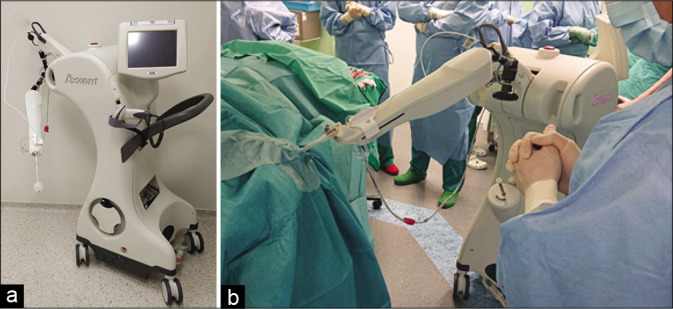
(a and b) An overview of the Axxent equipment (The Xoft® Electronic Brachytherapy (eBx®) System®, USA) (Left). The system at work in the operating room: the applicator balloon is connected to the system and the X-ray source has been introduced (Right).
Ethics
This study was approved by the local ethics committees and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its amendments.[50] Written informed consent was obtained from all subjects before screening.
Study procedures
At Moscow, 15 consecutive subjects with recurrent GBM underwent maximum safe microsurgical resection of their recurrent tumor in combination with IBEB (IBEB Group). These subjects received no further adjuvant treatment. In the same period, Novosibirsk also recruited 15 consecutive subjects with recurrent GBM. These subjects underwent the same maximally safe resection, followed by routine postoperative adjuvant chemotherapy with or without radiotherapy, based on investigator preference (Control Group).
GBM recurrences were confirmed by contrast-enhanced MRI perfusion and additional PET-CT with amino acid tracers in selected cases. In case of questionable results, intraoperative pathomorphological examination (frozen section analysis) was performed. Ultimately, the presence of tumor recurrence was confirmed by pathological examination after surgery.
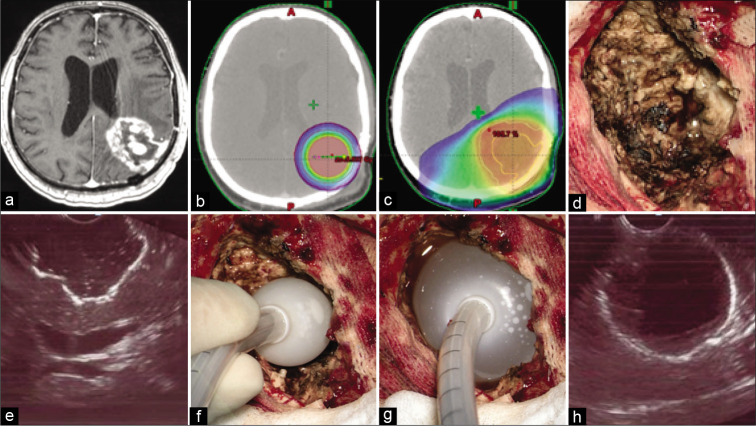
Preoperatively, the radiation oncologist, medical physicist, and neurosurgeon developed in all cases the IBEB plan (BrachyVision™; Varian Medical Systems, Inc., Palo Alto, CA). To provide this preoperative dosimetric estimate, reliable preoperative neuroimaging techniques were utilized to estimate the volume of the predicted post-operative surgical cavity and the appropriate balloon size to fit it. This allowed also the estimation of the IBEB X-ray source dose delivery to be applied [Figure 2a-c].
Figure 2:
(a) T1-weighted contrast-enhanced axial magnetic resonance imaging image demonstrates local glioblastoma recurrence prior to resection followed by intraoperative balloon electronic brachytherapy (IBEB). (b) IBEB isodose distribution based on computed tomography (CT) scans covers tumor bed sparing surrounding brain tissue. (c) External beam radiotherapy plan in comparison to IBEB. Isodose distribution based on the same CT scans affects extensively surrounding brain tissue which was irradiated after first surgery. (d) An overview of the post-resection cavity. (e) Intraoperative ultrasound demonstrates configuration of the postresection cavity which is free of macroscopic disease. (f) Deflated applicator balloon being introduced into the post-resection cavity. (g) Final position of the inflated applicator balloon with its dense adherence to the walls of the post-resection cavity. (h) Proper position of the balloon confirmed by intraoperative ultrasound.
Resection in both groups of subjects was conducted under general anesthesia, targeting maximal and safe resection under computed frameless MRI with contrast enhancement guidance, metabolic navigation with 5-aminolevulinic acid (5-ALA), and neurophysiological monitoring of evoked motor potentials. No “awake surgery” resection was conducted in the present patients. In the cases of multifocal tumor, only the largest neoplastic focus was resected, since it usually was responsible for the greatest mass effect. As described above IBEB was performed using the electronic brachytherapy device. After microsurgical removal of the neoplasm, the resection cavity was measured by filling it with isotonic sodium chloride solution. An empty IBEB applicator balloon was then introduced into this cavity and filled with the known volume of sodium chloride solution to give it a spherical shape, tightly adhering to the walls of the tumor bed. This was confirmed ultrasonographically [Figure 2d-h]. After the introduction of the IBEB X-ray source into the channel of the applicator balloon, a single dose of 20 Gy was delivered at the surface of the applicator balloon. The X-ray source was then removed from the channel of the balloon applicator. The isotonic sodium chloride solution was then aspirated to deflate and remove the balloon applicator. Tumor samples were sent for pathologic assessment, including histological, immunohistochemical (GFAP, p53), and molecular genetic (IDH1/2 mutation, MGMT methylation) studies. IDH 1/2 mutation was assessed using Sanger gene sequencing,[36] whereas MGMT promoter methylation status was assessed by real-time methylation-specific polymerase chain reaction.[51]
A baseline postoperative MRI scan with and without contrast was obtained within 24 h. Following IBEB, subjects did not receive any other adjuvant treatment except for those subjects with multifocal tumors, where additional stereotactic conformal fractionated irradiation was performed to treat the remaining lesions. Two patients in the IBEB group received additional surgery and IBEB treatment to deal with multifocal tumor growth. One subject with multifocal lesions (Nr. 10) did not receive any additional radiation therapy.
In the control group, 13 subjects received adjuvant therapy by preference of the treating neurosurgeon. The systemic treatment regimens included temozolomide, bevacizumab, lomustine, and mustophoran. Eight subjects received additional EBRT with doses between 36 and 60 Gy and one subject underwent another surgery.
Follow-up and analysis
All subjects were followed monthly according to the protocol. This included clinical visits and evaluation for performance status, toxicities, and clinical evidence of progression, with imaging controls every 3 months. This included MR imaging with and without contrast, perfusion-weighted MR to assess cerebral blood volume, and PET-CT with 18-FDOPA.
Contrast-enhanced tumor volume before and after resection was evaluated with automated volumetric analysis based on DICOM MR images (NeuroSegment Software; Novosibirsk, Russia).[23] A standardized assessment of possible adverse events including the development of radionecrosis in the IBEB area was performed using Common Terminology Criteria for Adverse Events (CTCAE, version 4.03).[30]
Study endpoints
This is a hypothesis-generating small pilot study with two contemporaneous cohorts enrolled simultaneously at two centers, without randomization. The endpoints included OS, local progression-free survival (LPFS) and the impact of the residual postsurgical tumor volume on OS and LPFF. OS was defined as the interval from the day of the recurrent GBM surgery to death for any reason or to the last documented follow-up, whichever occurred first; LPFS was defined as the time between surgery to any Local Progression/Tumor reoccurrence within 20 mm of the cavity margin or to the last documented follow-up, whichever occurred first. Based on the suggestion of the literature regarding the impact of the extent of residual tumor volume determined by MRI within 24 h after surgery on OS,[25,29,23,15] the impact of this variable was assessed using a cutoff point of 2.5 cm. Furthermore, the occurrence of treatment-related adverse events was recorded.
Statistical analysis
Statistical analyses of the treatment groups, including appropriate measures of central tendency and distribution, were performed using commercial software (IBM SPSS Statistics®; Armonk, NY and XLSTAT; Addinsoft, New York, NY). The effect of IBEB and the extent of tumor resection on OS and LPFS were assessed using Kaplan–Meier curves and the Log-rank test. A multivariate analysis (MANOVA) was carried out for both treatment groups. Independent variables evaluated for their impact on the efficacy endpoints included volume of residual disease, adjuvant therapy, gender, KPS, and IDH1/2 status. A univariate Cox proportional hazards model was used to calculate the hazard ratio (HR) for radical resection and IBEB therapy and a MANOVA was performed with corresponding adjustments for variables with potential confounding effects.
RESULTS
Baseline demographics and clinical characteristics
Thirty subjects were treated in the IBEB Group (Subjects 1-15) and Control Group (Subjects 16-30). Subjects in the IBEB Group had recurrent GBM (Grade IV, WHO 2016) with a mean age of 52.9 years (range, 40.0–71.0 years). Six (40%) were male. Median KPS was 90% (range, 60–100%). Other subject and tumor characteristics are summarized in [Table 1]. The follow-up period after IBEB ranged from 4.0 to 54.0 months as of March 31, 2021.
Table 1:
Magnetic resonance imaging and clinical characteristics of subjects that underwent IBEB.
The Control Group included 15 subjects with recurrent GBM (Grade IV, WHO 2016). Two subjects did not receive any further adjuvant therapy due to postoperative complications, but they were included in the intent-to-treat analysis. The mean age of the control group subjects was slightly younger at 48.6 years (range 24.0–74.0 years). The majority, (67.7%) were male unlike what observed in the IBEB Group. Subjects in the control group had a median KPS of 85.3% (range, 60–100%). Other subject and tumor characteristics are summarized in [Table 2]. Follow-up after re-resection ranged from 2.0–22.5 months.
Table 2:
Magnetic resonance imaging and clinical characteristics of subjects in the control arm.
Subjects in both treatment groups had a ≥6-month period between the last day of the initial radiation and any subsequent radiotherapy for the recurrent tumor [Tables 1 and 2]. Before being included in the IBEB Group, tumor resection had been performed once with subsequent adjuvant chemoradiotherapy (fractionated radiation therapy plus Temozolomide followed by maintenance Temozolomide) in 14 subjects; one subject had previously undergone two tumor resections and the relapse-free period after the second surgery was 8.0 months whit the time since the first surgery at inclusion was 38.0 months. For the entire cohort of 15 subjects, the median relapse-free period was 4.0 months (range, 1.0–18.0 months).
The location of the tumor resections is presented in [Table 1]. The mean (SD) of the preoperative contrast enhancing tumor volume (CEV) was 24.5 (18.6) cm3 (range 6.0–83.0 cm3).
Ten IBEB Group subjects had local tumor recurrence in the immediate vicinity of the resection cavity without signs of multifocal growth. In the remaining five (Patients 4, 5, 8, 10, and 11) cases, distant tumor growth was observed in addition to the main GBM focus. Mutation in the 132nd codon of the IDH1 was detected in three subjects. No subject presented with any mutation of the IDH2 gene. Analysis of the MGMT gene promoter methylation was performed in 11 subjects. The promotor was methylated in only three cases, one of which was in combination with the mutation of the IDH1 gene.
In 14 control group subjects, local tumor recurrence occurred in the immediate vicinity of the resection cavity without signs of multifocal growth. In one case with multifocal GBM (Subject 25), there was relapse at and distant to the site of operation. The median duration of the disease-free period after the initial surgery was 8.0 months (range 0–14 months). The localization of tumor foci, subjected to resection, is presented in [Table 2]. The mean (SD) tumor CEV undergoing resection was 37.4 (41.6) cm3 (range, 5.0–154.0 cm3). Four subjects displayed a codon 132 IDH1 mutation, two of which also had an IDH2 mutation and three also had methylation of MGMT promoter gene. In the remaining subjects, MGMT promoter gene was unmethylated.
IBEB and control arm results
The postoperative residual contrast-enhanced volume (PCEV) values of the main lesion for each subject in the IBEB group are shown in [Table 3]. The mean (SD) PCEV value of all subjects of the IBEB group is 3.2 (2.45) cm3 (range 0.1–7.0). The mean duration of an IBEB session was 11.8 min (range, 5.3–25.0 min). The mean volume of the balloon applicator was 49.3 cm3 (range, 20.0–120.0 cm3). Median LPFS for the entire IBEB group was 8.0 months (range, 4–54 months); in 67% of the IBEB patients the LPFS exceeded 6 months. One subject of the IBEB group was alive and did not have any signs of local tumor recurrence at the end of March 2021.
Table 3:
Duration and direct results of IBEB.
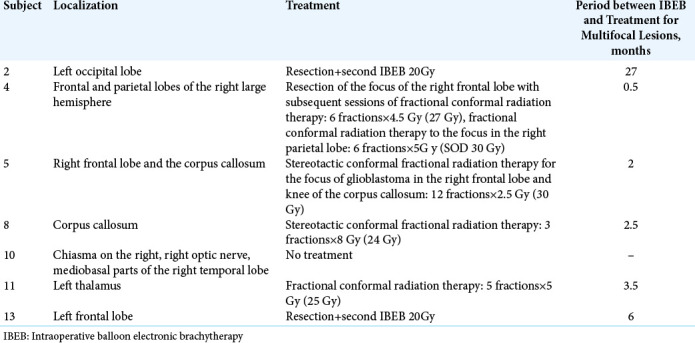
In a subgroup of subjects of the IBEB group (n = 7) with PCEV >2.5 cm3, the median LPFS was 5.0 months (range 3.5– 8.0 months). It should also be noted that in this subgroup, four subjects showed multifocal growth of their GBM. In the subgroup of subjects with PCEV ≤2.5 cm3 (n = 8), the median LPFS was 16.5 months (range 7.5–54.0 months). This subgroup included one subject with multifocal GBM. Whereas five (4, 5, 8, 10, and 11) patients experienced multifocal tumor growth before IBEB treatment, two patients (2 and 13) presented multifocal lesions after IBEB, outside the radiation treatment field. Patients with multifocal lesions in the IBEB group were treated after IBEB with EBRT, or surgery followed by a second course of IBEB. Therapeutic approaches used to control the multifocal tumors in the IBEB group are given in [Table 4].
Table 4:
Additional treatment for subjects in the IBEB arm who presented multifocal disease.
The PCEV values of all subjects of the control group are shown in [Table 5]. Mean (SD) PCEV was 6.2 cm3 (11.0) (range 0.6–45.0 cm3). After resection of the recurrent tumor, 13 subjects received adjuvant chemoradiation treatment. In eight of those 13 subjects, external fractional irradiation of 2 Gy per fraction was carried out in 30 fractions according to the European Organization of Research and Treatment of Cancer recommendations [Table 5]. In five subjects, adjuvant therapy consisted solely of chemotherapy with various agents, due to safety concerns about radiation therapy and individual patient’s decisions.
Table 5:
Results after second surgery, control arm.
The median LPFS for the entire Control Group (n = 15) was 6.0 months (range, 2.0–10.0 months) and 33% (n = 5) of these subjects had a LPFS >6 months. In a subgroup of subjects (n = 8) with PCEV >2.5 cm3, the median LPFS was 2.0 months (range, 2.0–6.0 months). In the subgroup of subjects with PCEV ≤2.5 cm3 (n = 7), the median LPFS was 8.0 months (range, 6.0–10.0 months).
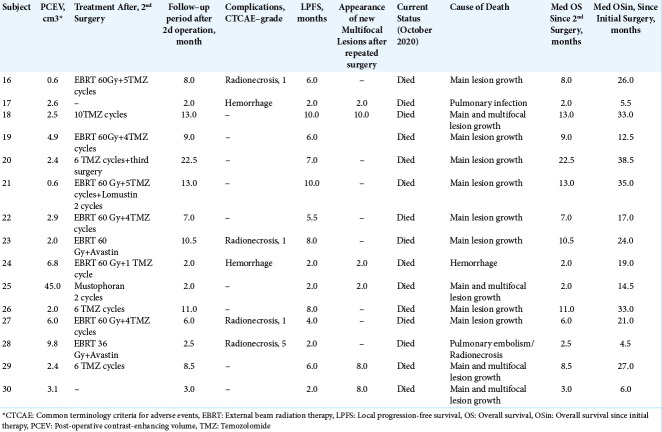
Median OS for the IBEB group was 11.0 months (range, 4.0–54.0 months). In the subgroup of subjects with PCEV >2.5 cm3 (n = 7), the median OS was 7.5 months (range, 4.0–11.0 months). In the subgroup of subjects with PCEV ≤2.5 cm3 (n = 8), median OS was 21.2 months (range, 7.5– 54.0 months). Relatively rapid progression was noted in all subjects with PCEV >2.5 cm3, irrespective of multifocal tumor growth. The Kaplan–Meier analysis of OS after reresection of recurrent GBM in combination with IBEB is shown in [Figure 3]. A statistically significant difference was found between survival in the two subgroups stratified according to PCEV (Log Rank χ2 = 8.03, P = 0.005, P < 0.05). The median OSin for the IBEB group since the initial surgery was 29.5 months (range, 17.0–69.0 months).
Figure 3:
Kaplan-Meier curves for overall survival in the intraoperative balloon electronic brachytherapy group stratified according to postoperative residual contrast-enhanced volume (PCEV): the subgroup of subjects with PCEV >2.5 cm3 marked in red, the subgroup of subjects with PCEV ≤2.5 cm3 marked in green; log rank χ2 = 8.03, P = 0.005, P < 0.05.
Median OS for the entire control group of subjects (n = 15) was 8.0 months (range, 2.0–22.5 months). In the subgroup of subjects with PCEV >2.5 cm3, median OS was 2.8 months (range, 2.0–9.0 months). In the subgroup of subjects with PCEV ≤2.5 cm3, median OS was 11.0 months (range, 8.0– 22.5 months). The median OSin after initial surgery for the control group was 21.0 months (range, 4.5–38.5 months) [Table 5].
Treatment group comparisons
Data analysis of the CEV and PCEV, KPS, MGMT in these groups showed a normal distribution of values and the equality of variances (Livin criterion for dispersions equality, P > 0.05). Analysis of variance testing did not reveal statistically significant differences between IBEB and control groups (Pillai multivariate trace = 0.153, P = 0.390).
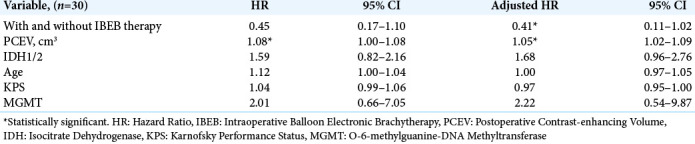
The results of a univariate Cox proportional hazards model to calculate the HR for PCEV and IBEB therapy are listed in [Table 6]. There were no statistically significant differences in HR according to the parameters IDH1/2, age, KPS, and MGMT. IBEB group subjects had a longer survival than subjects in the Control group. The postoperative risk of death was associated with an increase in PCEV.
Table 6:
Univariate and multivariate cox proportional hazards analysis for the entire subject group.
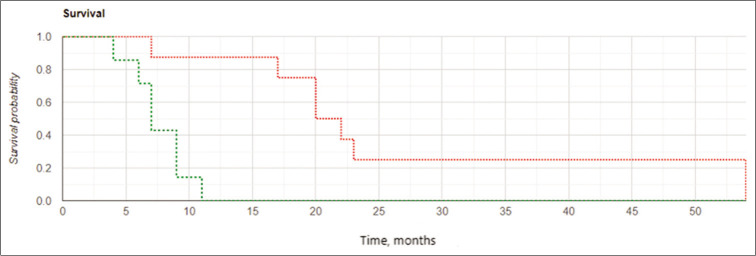
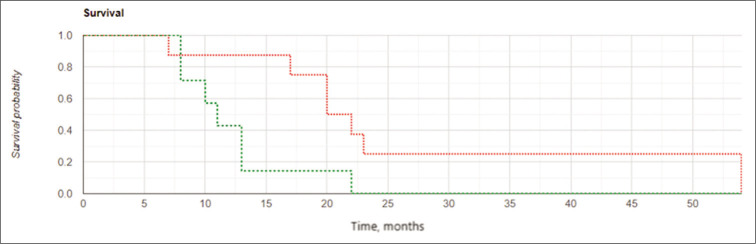
Among subjects in the IBEB, both the median LPFS and the median OS were significantly higher than those in control group (8.0 vs. 6.0 months and 11.0 vs. 8.0 months, respectively). The Kaplan–Meier curve confirmed statistically significant increased OS and LFPS for the IBEB group of subjects compared to the control group (OS: log rank χ2 = 4.23, P = 0.04, P < 0.05; LPFS: log rank χ2 = 4.93, P = 0.026, P < 0.05) [Figures 4 and 5].
Figure 4:
Kaplan-Meier curves for overall survival: The intraoperative balloon electronic brachytherapy group marked in green, the control group marked in red; Log Rank χ2 = 4.23, P = 0.04, P < 0.05.
Figure 5:
Kaplan-Meier curves for local progression-free survival: the intraoperative balloon electronic brachytherapy group marked in green, the control group marked in red, Log Rank χ2 = 4.93, P = 0.026, P < 0.05.
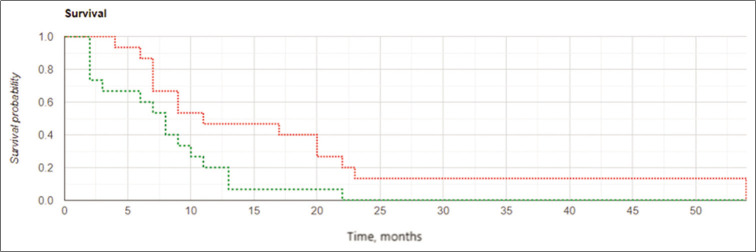
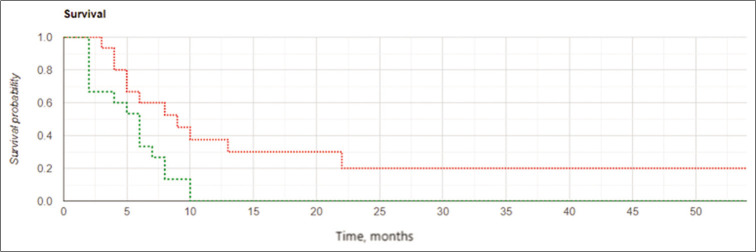
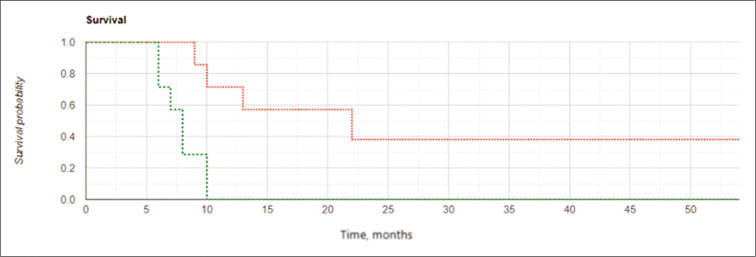
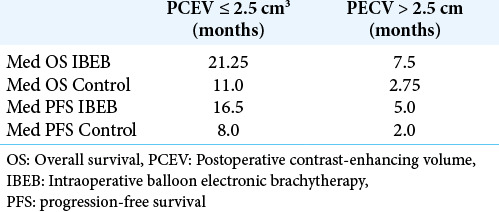
The results of the study confirmed the important role of the extent of tumor resection in cases of GBM recurrence. Kaplan– Meier curves [Figures 6 and 7] demonstrated better results in OS and LPFS for the IBEB subgroup of subjects having PCEV ≤2.5 cm3 compared to the same control cohort with medians 21.25 (OS) and 16.5 (LPFS) months for IBEB versus 11.0 (OS) and 8.0 (LPFS) months for Control, respectively (OS: Log Rank χ2 = 4.13, P = 0.042, P < 0.05; LPFS: Log Rank χ2 = 0.24, P = 0.007, P < 0.05). In addition, for both the IBEB and the control groups, the median LPFS and median OS in the subgroups having PCEV ≤2.5 cm3 were significantly longer than the subgroups having PCEV >2.5 cm3 [Table 7].
Figure 6:
Kaplan-Meier curves for overall survival in the subgroups of subjects with postoperative residual contrast-enhanced volume ≤2.5 cm3: the intraoperative balloon electronic brachytherapy group marked in green, the control group marked in red; Log Rank χ2 = 4.13, P = 0.042, P < 0.05.
Figure 7:
Kaplan-Meier curves for local progression-free survival in the subgroups of subjects with postoperative residual contrast-enhanced volume ≤2.5 cm3: the intraoperative balloon electronic brachytherapy group marked in green, the control group marked in red; Log Rank χ2 = 7.24, P = 0.007, P < 0.05.
Table 7:
Med OS and med LPFS based on PECV size in IBEB and control groups.
DISCUSSION
The treatment of recurrent GBM is still matter of debate. The role of re-resection has become widely accepted in the last decade provided some clinical parameters would seem to justify this, including age, relatively good Karnofsky status and no invasion of functionally relevant areas.[31]
However, others factors such as MGMT methylation, IDH 1/2 mutation, and comorbidities are known to play a role in the outcome of GBM patients. This means that evaluation of newly proposed therapeutic protocols requires unavailable extreme caution, and preliminary though encouraging results, if convincing, should be considered only a suggestive base for properly designed future studies.
In our study, we proposed for the first time the use of IBEB technique in GBM patients. This technique was recently introduced and has become a widely accepted form of local radiation therapy for malignant tumors of several organs which seems to offer the advantage of increasing the radiation dosage delivered to the malignancy while sparing almost completely the surrounding healthy tissue. We designed an open-label, not randomized study because the IBEB technology was available only in one center. For the recruitment of the control group of patients, another independent center was chosen to eliminate bias. However, the two institutions share several members of the medical staff and have strictly similar treatment protocols for GBM patients. In particular, criteria for reoperation were absolutely the same, and postoperative management in no-IBEB group of patients followed the most updated therapeutic recommendations.
The present results indicate improved, LPES in the IBEB treated group which reached statistical significance (8.0 months vs. 6.0 months) which appeared also more evident in the cases in which near-total removal had been achieved (16.5 months vs. 8.0 months). Consequently also OS was significantly longer in the IBEB group.
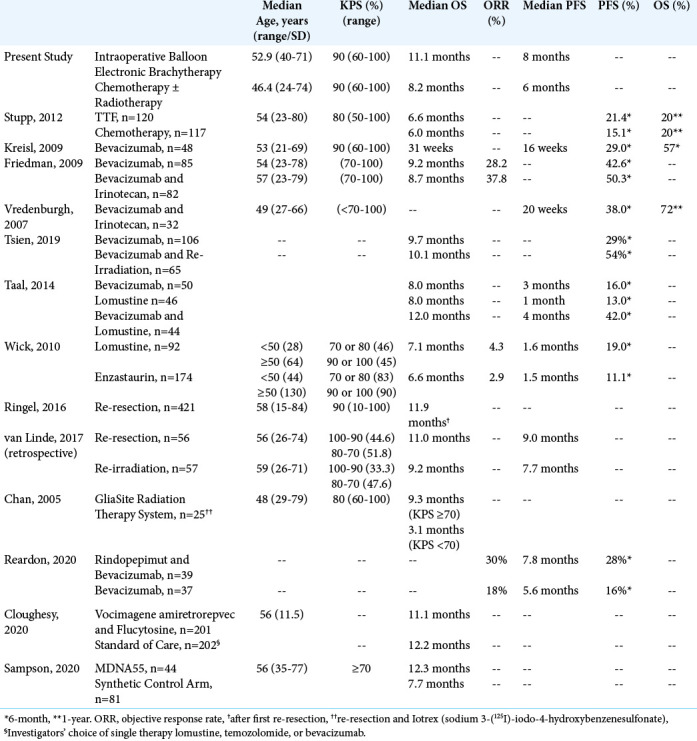
We collected from the relevant literature previously published outcomes data for the treatment of recurrent GBMs. These are summarized in [Table 8]. Obviously, direct comparisons are out of consideration, since each trial possessed its own selection parameters. The median OS among subjects undergoing re-resection for recurrent GBM was 11.9 months.[33] An engineered interleukin 4 (IL-4) fused to pseudomonas exotoxin A (MDNA55) was administered to subjects with recurrent GBM using convection-enhanced delivery to bypass the blood-brain barrier[35] and the median OS was 11.6 months, similar to the present IBEB patients group. Survival was highest among subjects expressing IL4 Receptor (IL4R) and those with unmethylated MGMT. As radiation-induced IL4 and IL4 R overexpression have been reported in human cancer cells, a potential future combination of local MDNA55 administration with IBEB treatment might perhaps be able to prevent aggressive tumor behavior and at least slow post-radiation tumor recurrence.[19] In a recent study comparing vocimagene amiretrorepvec (Tocca 511) plus flucytosine versus standard of care with lomustine, temozolomide, or bevacizumab,[6] the median OS was 11.1 and 12.2 months, respectively. However, in this study subjects with multifocal lesions were excluded while some cases of anaplastic astrocytoma were also included. Other therapies appear to be generally less successful especially for subjects who would not be eligible for re-resection. Subjects with recurrent GBM treated with bevacizumab achieved a median OS of approximately 8–10 months.[11,20,42,45] Combining bevacizumab with irinotecan, lomustine, and re-radiation only extended median OS to approximately 10– 12 months.[11,42, 45] Similarly, subjects treated with lomustine for recurrent GBM achieved a median OS of 7–8 months. [42,49] The median OS following TTF was only 6 months.[41] Among subjects treated with a balloon catheter device using a high dose of a liquid radiation source (GliaSite Radiation Therapy System, RTS),[4] the median OS was 9.1 months.
Table 8:
Treatments for Recurrent Glioblastoma
Our study reports two cases of radionecrosis in the IBEB Group with only one CTCAE Grade 3 toxicity. No other grade ≥3 adverse events occurred in the IBEB group. This is in the same range as RTOG 1205, which reported 5% grade ≥3 events.[45] The RTS trial reported a wound infection in one subject, symptomatic radionecrosis in two subjects and a neurologic deficit (transient expressive aphasia) in one subject.[4]
In our patients the IBEB treatment took approximately 30 min, with a duration of <20 min in the vast majority of the patients, thus IBEB treatment did not significantly increase the total surgery time [Table 3] and had no immediate postoperative complications. Despite the fact that all subjects had previously received external irradiation with total boost dose of 60 Gy, the development of clinically significant radiation necrosis in the IBEB-treated group was observed in only two of patients (13.3%) during the first 6 months after treatment. These subjects did not have clear predictors for these radiation-related complications. In the control group, there were twice as many (four subjects) cases of radiation necrosis (26.7%) with one fatal outcome, which may potentially be associated with small intervals between external irradiation for a newly diagnosed tumor and its recurrence. None of the patients suffered infection or CSF fistula postoperatively.
The effect of any adjuvant therapy decreases with the amount of residual tumor. GBM is an aggressively growing tumor, with the potential to increase substantially in volume within a short period.[14,27] Delivering radiation at the time of surgery (when tumor mass is minimized) could potentially increase the effect of the IORT compared to a radiation regimen starting weeks after surgery.
As mentioned previously, metabolic guidance using 5-ALA was used intraoperatively. Protoporphyrin IX (PpIX, a 5-ALA metabolite) selectively accumulates in cancer cells and was characterized as a radio-responsive compound. As in vitro studies and in vivo studies in small animals have shown, PpIX produces reactive oxygen species upon X-ray irradiation, which induces DNA double-strand breaks resulting in cell cycle arrest.[43,52,53] Future investigations should confirm this radio-sensitizing effect and its potential positive impact on IBEB results in subjects with recurrent GBM.
We did not operate any of the present patients on awake surgery, a treatment protocol of which we have extensive experience particularly in recurrent GBM. Awake surgery would give immediate, functional control to the operating surgeon, and might encourage more aggressive, thought safe, surgical conduct. However, highly developed technology such as sophisticate neuromonitoring and metabolic navigation in anesthetized patients can offer equivalent safety standards to the operating surgeon who is so encouraged to perform maximally aggressive though safe resection.
Study limitations include an open-label study design, inability to control for all the factors contributing to outcome, inclusion of subjects with multifocal disease, sample size, and limited follow-up period. In addition, patients were not allocated randomly to either treatment regiments as they were treated in two different Institutions. However, we believe that the present results are appealing and should encourage further investigations by properly designed clinical trials in order determine if the innovative IBEB protocol which we used in this study might be helpful to other subjects with similar characteristics.
CONCLUSION
The results of this prospective, two-center, and comparative cohort pilot study suggest that a significant improvement in median LPFS and OS may occur in subjects undergoing IBEB following repeated resection of recurrent GBM in comparison with a control group who received standard adjuvant chemo-radiotherapy following re-resection. IBEB was associated with manageable toxicity. Subjects with a PCEV <2.5 cm3 and free of concurrent multifocal disease showed particular benefit from IBEB. This first comparative study, while limited by small sample size and its open-label nature, provides hypothesis-generating data that may warrant further investigation on the potential use of IBEB in malignant gliomas and its risk/benefit/ratio.
Acknowledgment
The authors acknowledge the editorial assistance of Dr. Carl S. Hornfeldt, Apothekon, Inc., during the preparation of this manuscript.
Footnotes
How to cite this article: Krivoshapkin A, Gaytan A, Abdullaev O, Salim N, Sergeev G, Marmazeev I, et al. Prospective comparative study of intraoperative balloon electronic brachytherapy versus resection with multidisciplinary adjuvant therapy for recurrent glioblastoma. Surg Neurol Int 2021;12:517.
Contributor Information
Aleksey Krivoshapkin, Email: alkr01@yandex.ru.
Aleksey Gaytan, Email: lanceter@mail.ru.
Orkhan Abdullaev, Email: oabdullaev@emcmos.ru.
Nidal Salim, Email: nsalim@emcmos.ru.
Gleb Sergeev, Email: gsergeev@emcmos.ru.
Ilya Marmazeev, Email: imarmazeev@emcmos.ru.
Evaldas Cesnulis, Email: info@cesnulis.ch.
Tim Killeen, Email: tim.killeen@doctors.org.uk.
Vladimir Tyuryn, Email: vladimir@jetology.aero.
Roman Kiselev, Email: rayjelly@gmail.com.
Pavel Syomin, Email: syominp@yandex.ru.
Aldo Spallone, Email: aldospallone@hotmail.com.
Declaration of patient consent
Institutional Review Board (IRB) permission obtained for the study.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Adkison JB, Tomé W, Seo S, Richards GM, Robins HI, Rassmussen K, et al. Reirradiation of large-volume recurrent glioma with pulsed reduced-dose-rate radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79:835–41. doi: 10.1016/j.ijrobp.2009.11.058. [DOI] [PubMed] [Google Scholar]
- 2.Barney C, Shukla G, Bhamidipati D, Palmer JD. Re-irradiation for recurrent glioblastoma multiforme. Chin Clin Oncol. 2017;6:36. doi: 10.21037/cco.2017.06.18. [DOI] [PubMed] [Google Scholar]
- 3.Cabrera AR, Cuneo KC, Desjardins A, Sampson JH, McSherry F, Herndon JE, 2nd, et al. Concurrent stereotactic radiosurgery and bevacizumab in recurrent malignant gliomas: A prospective trial. Int J Radiat Oncol Biol Phys. 2013;86:873–9. doi: 10.1016/j.ijrobp.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 4.Chan TA, Weingart JD, Parisi M, Hughes MA, Olivi A, Borzillary S, et al. Treatment of recurrent glioblastoma multiforme with GliaSite brachytherapy. Int J Radiat Oncol Biol Phys. 2005;62:1133–9. doi: 10.1016/j.ijrobp.2004.12.032. [DOI] [PubMed] [Google Scholar]
- 5.Clarke J, Neil E, Terziev R, Gutin P, Barani I, Kaley T, et al. Multicenter, phase 1, dose escalation study of hypofractionated stereotactic radiation therapy with bevacizumab for recurrent glioblastoma and anaplastic astrocytoma. Int J Radiat Oncol Biol Phys. 2017;99:797–804. doi: 10.1016/j.ijrobp.2017.06.2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cloughesy TF, Petrecca K, Walbert T, Butowski N, Salacz M, Perry J, et al. Effect of vocimagene amiretrorepvec in combination with flucytosine vs standard of care on survival following tumor resection in patients with recurrent high-grade glioma: A randomized clinical trial. JAMA Oncol. 2020;12:e203161. doi: 10.1001/jamaoncol.2020.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.D’Amico RS, Englander ZK, Canoll P, Bruce JN. Extent of resection in glioma-a review of the cutting edge. World Neurosurg. 2017;103:538–49. doi: 10.1016/j.wneu.2017.04.041. [DOI] [PubMed] [Google Scholar]
- 8.Eljamel S. 5-ALA fluorescence image guided resection of glioblastoma multiforme: A meta-analysis of the literature. Int J Mol Sci. 2015;16:10443–56. doi: 10.3390/ijms160510443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Epstein MS, Silverstein MJ, Lin K, Kim B, de Leon C, Khan S, et al. Acute and chronic complications in patients with ductal carcinoma in situ treated with intraoperative radiation therapy. Breast J. 2016;22:630–6. doi: 10.1111/tbj.12650. [DOI] [PubMed] [Google Scholar]
- 10.Fogh SE, Andrews DW, Glass J, Curran W, Glass C, Champ C, et al. Hypofractionated stereotactic radiation therapy: An effective therapy for recurrent high-grade gliomas. J Clin Oncol. 2010;28:3048–53. doi: 10.1200/JCO.2009.25.6941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedman HS, Prados MD, Wen PY, Mikkelsen T, Schiff D, Abrey LE, et al. Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol. 2009;27:4733–40. doi: 10.1200/JCO.2008.19.8721. [DOI] [PubMed] [Google Scholar]
- 12.Gabayan AJ, Green SB, Sanan A, Jenrette J, Schultz C, Papagikos M, et al. GliaSite brachytherapy for treatment of recurrent malignant gliomas: A retrospective multi-institutional analysis. Neurosurgery. 2006;58:701–9. doi: 10.1227/01.NEU.0000194836.07848.69. [DOI] [PubMed] [Google Scholar]
- 13.Gallia GL, Brem S, Brem H. Local treatment of malignant brain tumors using implantable chemotherapeutic polymers. J Natl Compr Canc Netw. 2005;3:721–8. doi: 10.6004/jnccn.2005.0042. [DOI] [PubMed] [Google Scholar]
- 14.Goyal U, Pan J, Cui H, Stea B. Does ultrasound measurement improve the accuracy of electronic brachytherapy in the treatment of superficial non-melanomatous skin cancer? J Contemp Brachytherapy. 2017;9:14–9. doi: 10.5114/jcb.2017.65476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grabowski MM, Recinos PF, Nowacki AS, Schroeder JL, Angelov L, Barnett GH, et al. Residual tumor volume versus extent of resection: Predictors of survival after surgery for glioblastoma. J Neurosurg. 2014;121:1115–23. doi: 10.3171/2014.7.JNS132449. [DOI] [PubMed] [Google Scholar]
- 16.Gutin PH, Iwamoto FM, Beal K, Mohile NA, Karimi S, Hou BL, et al. Safety and efficacy of bevacizumab with hypofractionated stereotactic irradiation for recurrent malignant gliomas. Int J Radiat Oncol Biol Phys. 2009;75:156–63. doi: 10.1016/j.ijrobp.2008.10.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hervey-Jumper SL, Berger MS. Maximizing safe resection of low-and high-grade glioma. J Neurooncol. 2016;130:269–82. doi: 10.1007/s11060-016-2110-4. [DOI] [PubMed] [Google Scholar]
- 18.Kazmi F, Soon YY, Leong YH, Koh WY, Vellayappan B. Reirradiation for recurrent glioblastoma (GBM): A systematic review and meta-analysis. J Neurooncol. 2019;142:79–90. doi: 10.1007/s11060-018-03064-0. [DOI] [PubMed] [Google Scholar]
- 19.Kim ES, Choi YE, Hwang SJ, Han YH, Park MJ, Bae IH. IL-4, a direct target of miR-340/429, is involved in radiation-induced aggressive tumor behavior in human carcinoma cells. Oncotarget. 2016;7:86836–56. doi: 10.18632/oncotarget.13561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kreisl TN, Kim L, Moore K, Duic P, Royce C, Stroud I, et al. Phase II trial of single-agent bevacizumab followed by bevacizumab plus irinotecan at tumor progression in recurrent glioblastoma. J Clin Oncol. 2009;27:740–5. doi: 10.1200/JCO.2008.16.3055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Krivoshapkin A, Gaytan A, Salim N, Abdullaev O, Sergeev G, Marmazeev I, et al. Repeat resection and intraoperative radiotherapy for malignant gliomas of the brain: A history and review of current techniques. World Neurosurg. 2019;132:356–62. doi: 10.1016/j.wneu.2019.09.037. [DOI] [PubMed] [Google Scholar]
- 22.Krivoshapkin AL, Sergeev GS, Gaytan AS, Kalneus LE, Kurbatov VP, Abdullaev OA, et al. Automated volumetric analysis of postoperative magnetic resonance imaging predicts survival in patients with glioblastoma. World Neurosurg. 2019;126:e1510–7. doi: 10.1016/j.wneu.2019.03.142. [DOI] [PubMed] [Google Scholar]
- 23.Krivoshapkin AL, Sergeev GS, Gaytan AS, Kurbatov VP, Kiselev RS, Kalneus LE. Impact of pre-and postoperative tumor volumetric data on survival of patients with glioblastomas. Bull J Neurol Psychiatr Neurosurg. 2016;6:59–67. [Google Scholar]
- 24.Krivosheya D, Prabhu SS, Weinberg JS, Sawaya R. Technical principles in glioma surgery and preoperative considerations. J Neurooncol. 2016;130:243–52. doi: 10.1007/s11060-016-2171-4. [DOI] [PubMed] [Google Scholar]
- 25.Li YM, Suki D, Hess K, Sawaya R. The influence of maximum safe resection of glioblastoma on survival in 1229 patients: Can we do better than gross-total resection? J Neurosurg. 2016;124:977–88. doi: 10.3171/2015.5.JNS142087. [DOI] [PubMed] [Google Scholar]
- 26.Lloyd SA, Rahn DA, Hoisak JD, Dragojević I. Evaluation of effective treatment depth in skin cancer treatments with electronic brachytherapy. Brachytherapy. 2018;17:990–4. doi: 10.1016/j.brachy.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 27.Louis DN, Perry A, Reifenberger G, Deimling A, FigarellaBranger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: A summary. Acta Neuropathol. 2016;131:803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 28.Lozares-Cordero S, Font-Gómez JA, Gandía-Martínez A, Miranda-Burgos A, Méndez-Villamón A, Villa-Gazulla D, et al. Treatment of cervical cancer with electronic brachytherapy. J Appl Clin Med Phys. 2019;2:78–86. doi: 10.1002/acm2.12657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Molinaro AM, Hervey-Jumper S, Morshed RA, Young J, Han SJ, Chunduru P, et al. Association of maximal extent of resection of contrast-enhanced and non-contrast-enhanced tumor with survival within molecular subgroups of patients with newly diagnosed glioblastoma. JAMA Oncol. 2020;6:495–503. doi: 10.1001/jamaoncol.2019.6143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Cancer Institute US National Institutes of Health. Common Terminology Criteria for Adverse Events (CTCAE Version 4.03) 2020. Available from: https://www.ctep.cancer.gov/protocoldevelopment/electronic_applications/ctc.htm [Last accessed on 2020 Oct 30]
- 31.National Comprehensive Cancer Network NCCN Guidelines Version 2. 2020 Anaplastic Gliomas, Glioblastoma, Recurrence. 2020. Available from: https://www.nccn.org/professionals/physician_gls/default.aspx [Last accessed on 2020 Nov 25]
- 32.Rapp M, Baernreuther J, Turowski B, Steiger HJ, Sabel M, Kamp MA. Recurrence pattern analysis of primary glioblastoma. World Neurosurg. 2017;103:733–40. doi: 10.1016/j.wneu.2017.04.053. [DOI] [PubMed] [Google Scholar]
- 33.Ringel F, Pape H, Sabel M, Krex D, Bock HC, Misch M, et al. Clinical benefit from resection of recurrent glioblastomas: Results of a multicenter study including 503 patients with recurrent glioblastomas undergoing surgical resection. Neuro Oncol. 2016;18:96–104. doi: 10.1093/neuonc/nov145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salim N, Krivoshapkin AL, Gaytan AS, Marmazeev IV, Abdullaev O, Sergeev GS, et al. The use of intraoperative radiation therapy in patients with recurrent gliomas of high malignancy. J Malignant Tumors. 2017;3:110. [Google Scholar]
- 35.Sampson JH, Achrol A, Aghi MK, Bankiewicz KS, Bexon M, Brem S, et al. MDNA55 survival in recurrent glioblastoma (rGBM) patients expressing the interleukin-4 receptor (IL4R) as compared to a matched synthetic control. J Clin Oncol. 2020;38:2513–3. [Google Scholar]
- 36.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–7. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scharfen CO, Sneed PK, Wara WM, Larson DA, Phillips TL, Prados MD, et al. High activity iodine-125 interstitial implant for gliomas. Int J Radiat Oncol Biol Phys. 1992;24:583–91. doi: 10.1016/0360-3016(92)90702-j. [DOI] [PubMed] [Google Scholar]
- 38.Silverstein MJ, Epstein MS, Lin K, Chen P, Khan S, Snyder L, et al. Intraoperative radiation using low-kilovoltage X-rays for early breast cancer: A single site trial. Ann Surg Oncol. 2017;24:3082–7. doi: 10.1245/s10434-017-5934-z. [DOI] [PubMed] [Google Scholar]
- 39.Stummer W, Novotny A, Stepp H, Goetz C, Bise K, Reulen HJ. Fluorescence-guided resection of glioblastoma multiforme by using 5-aminolevulinic acid-induced porphyrins: A prospective study in 52 consecutive patients. J Neurosurg. 2000;93:1003–13. doi: 10.3171/jns.2000.93.6.1003. [DOI] [PubMed] [Google Scholar]
- 40.Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 41.Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: A randomised phase III trial of a novel treatment modality. Eur J Cancer. 2012;48:2192–202. doi: 10.1016/j.ejca.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 42.Taal W, Oosterkamp HM, Walenkamp AM, Dubbink HJ, Beerepoot LV, Hanse MC, et al. Single-agent bevacizumab or lomustine versus a combination of bevacizumab plus lomustine in patients with recurrent glioblastoma (BELOB trial): A randomised controlled phase 2 trial. Lancet Oncol. 2014;15:943–53. doi: 10.1016/S1470-2045(14)70314-6. [DOI] [PubMed] [Google Scholar]
- 43.Takahashi J, Nagasawa S, Ikemoto MJ, Sato C, Sato M, Iwahashi H. Verification of 5-aminolevurinic radiodynamic therapy using a murine melanoma murine metastasis model. Int J Mol Sci. 2019;20:5155. doi: 10.3390/ijms20205155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatter SB, Shaw EG, Rosenblum ML, Karvelis KC, Kleinberg L, Weingart J, et al. An inflatable balloon catheter and liquid 125I radiation source (GliaSite radiation therapy system) for treatment of recurrent malignant glioma: Multicenter safety and feasibility trial. J Neurosurg. 2003;99:297–303. doi: 10.3171/jns.2003.99.2.0297. [DOI] [PubMed] [Google Scholar]
- 45.Tsien C, Pugh S, Dicker AP, Raizer JJ, Matuszak MM, Lallana E, et al. Randomized Phase II trial of re-irradiation and concurrent bevacizumab versus bevacizumab alone as treatment for recurrent glioblastoma (NRG Oncology/RTOG 1205): Initial outcomes and RT plan quality report. Int J Radiat Oncol Biol Phys. 2019;105:S78. [Google Scholar]
- 46.van Linde ME, Brahm CG, de Witt Hamer PC, Reijneveld JC, Bruynzeel AM, Vandertop WP, et al. Treatment outcome of patients with recurrent glioblastoma multiforme: A retrospective multicenter analysis. J Neurooncol. 2017;135:183–92. doi: 10.1007/s11060-017-2564-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people-a randomised study. Acta Neurochir (Wien) 2003;145:5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 48.Westphal M, Hilt DC, Bortey E, Delavault P, Olivares R, Warnke PC, et al. A phase 3 trial of local chemotherapy with biodegradable carmustine (BCNU) wafers (Gliadel wafers) in patients with primary malignant glioma. Neuro Oncol. 2003;5:79–88. doi: 10.1215/S1522-8517-02-00023-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wick W, Puduvalli VK, Chamberlain MC, van den Bent MJ, Carpentier AF, Cher LM, et al. Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol. 2010;28:1168–74. doi: 10.1200/JCO.2009.23.2595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.World Medical Association World medical association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- 51.Yachi K, Watanabe T, Ohta T, Fukushima T, Yoshino A, Ogino A, et al. Relevance of MSP assay for the detection of MGMT promoter hypermethylation in glioblastomas. Int J Oncol. 2008;33:469–75. [PubMed] [Google Scholar]
- 52.Yamada K, Murayama Y, Kamada Y, Arita T, Kosuga T, Konishi H, et al. Radiosensitizing effect of 5-aminolevulinic acid in colorectal cancer in vitro and in vivo. Oncol Lett. 2019;17:5132–8. doi: 10.3892/ol.2019.10198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang Q, Xiao G, Sun Q, Zeng J, Mu X, Chen L, et al. Radio-dynamic therapy (RDT) which combines cerenkov-induced PDT and RT. Int J Radiat Oncol Biol Phys. 2018;102:E506. [Google Scholar]