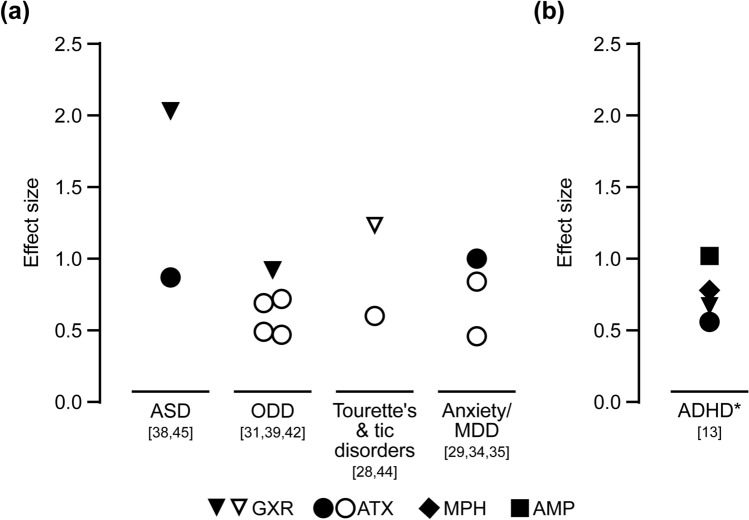
Fig. 2.
Summary of effect sizes from a included studies for which the effect size (standardized mean difference) was reported [28, 29, 31, 34, 35, 39, 42, 44, 45] or could be calculated [38, 42] for the effect of treatment compared with placebo on ADHD symptoms (ADHD-RS-IV total scores) in children and adolescents with ADHD and at least one of the prespecified comorbidities, and b as reported by Cortese et al. [13] for analyses where studies of children with psychiatric and neurological comorbidities were not excluded. Asterisk indicates that the sensitivity analysis suggested that the presence of psychiatric comorbidities did not significantly affect the results when studies of children with psychiatric and neurological comorbidities were excluded. Filled symbols indicate investigator-rated ADHD-RS-IV scores; open symbols indicate parent– or teacher–investigator-rated ADHD-RS-IV scores. ADHD attention-deficit hyperactivity disorder, ADHD-RS-IV ADHD Rating Scale IV, AMP amphetamine, ASD autism spectrum disorder, ATX atomoxetine, GXR guanfacine extended-release, MDD major depressive disorder, MPH methylphenidate, ODD oppositional defiant disorder