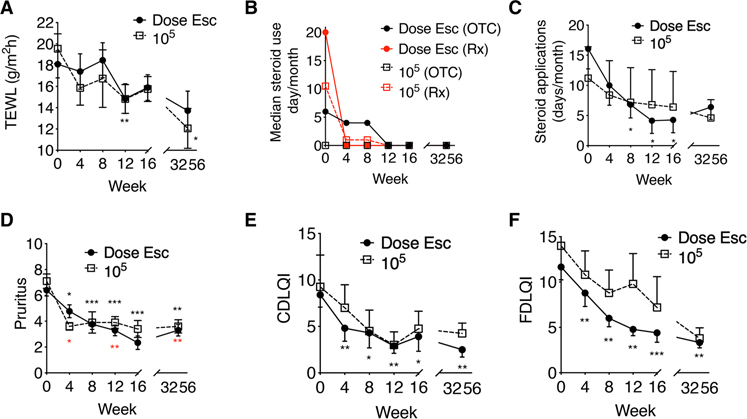
Fig. 2. Topical R. mucosa treatment is associated with improvements in AD.
(A) Mean values for trans-epidermal water loss (TEWL) during R. mucosa treatment and washout phase. (B and C) Median steroid cream applications per month for (B) over the counter (OTC) and prescription (Rx) topical steroid treatments and (C) mean values for combined steroid treatments [n = 16 for both (B) and (C)]. All topical steroid usage was reported as stable for months to years before enrollment of all patients with AD. Week 0 steroid usage reflects reported average steroid use over the 4 months before study enrollment. (D) Mean pruritus scores and (E and F) quality of life indices for study participants with AD treated with R. mucosa. (E and F) Children daily life quality index (CDLQI) (E) and (F) family daily life quality index (FDLQI); higher numbers indicate greater burden. Black asterisks are for dose escalation cohort, and red asterisks are for 105 CFU/body site dose. *P < 0.05, **P < 0.01, ***P < 0.001 versus enrollment value by paired ANOVA with Dunnett correction.