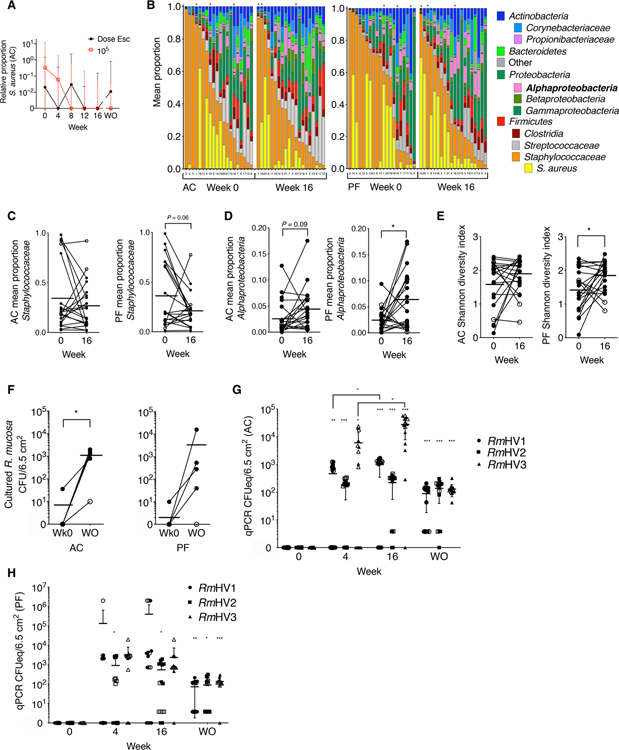
Fig. 3. Topical R. mucosa treatment is associated with long-term skin colonization.
(A) Shown are median ± interquartile range for relative abundance of S. aureus versus coagulase-negative Staphylococci in the antecubital fossa (AC) of patients with AD treated with R. mucosa at escalating doses (Dose Esc) or a 105 CFU/body site dose. (B) 16S rRNA sequencing of the skin microbiome in swabs from AC and popliteal fossa (PF) on weeks 0 and 16 of dose escalation. Columns are labeled by patient enrollment number but sorted by relative abundance of total Staphylococcaceae; black asterisks indicate patients who did not respond to R. mucosa treatment. (C to E) Relative proportions of indicated taxa (C and D) and Shannon diversity index (E) calculated from rRNA sequencing data in (B) for skin swabs from the AC and PF fossae; open circles represent nonresponding patients. (F) Culture-based enumeration of R. mucosa from antecubital and popliteal fossae (AC and PF) for the first five pediatric patients treated with R. mucosa at week 0 (Wk0) and at the washout visit (WO); open circles represent nonresponding patients. (G and H) Colony-forming unit equivalents (CFUeq) of R. mucosa from healthy volunteers (RmHV) measured by RTqPCR from skin swabs from AC (G) and PF (H) body sites (n = 15 each) are shown. Closed symbols represent the dose escalation cohort; open symbols represent the 105 CFU/body site dose cohort. *P < 0.05, **P < 0.01, and ***P < 0.001 by paired ANOVA with Dunnett correction versus week 0 value unless indicated.