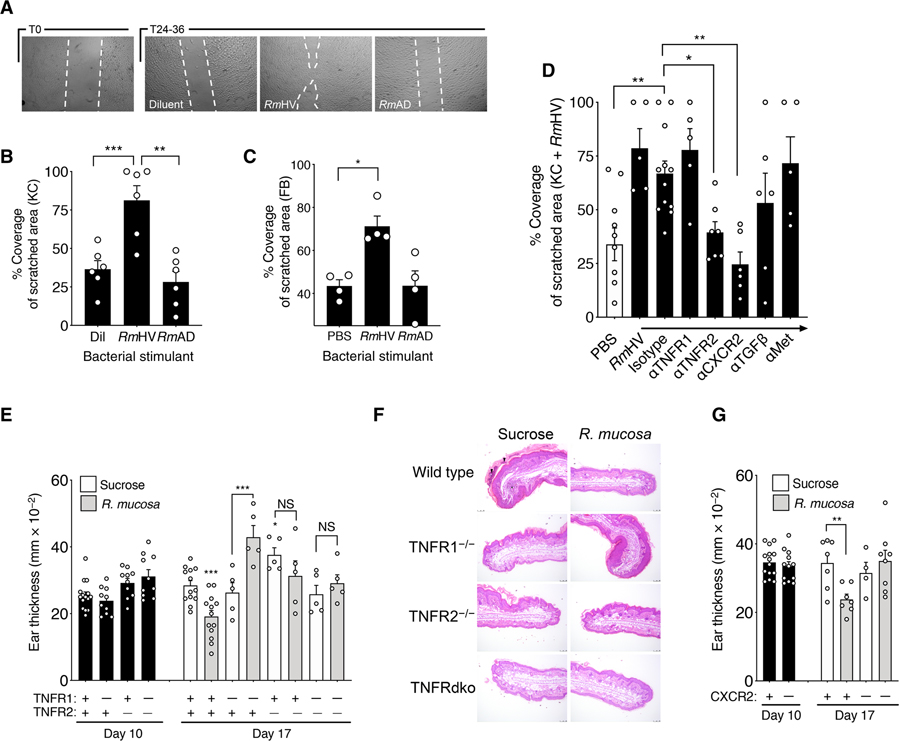
Fig. 5. R. mucosa activity was dependent on TNFR signaling.
(A) Shown are representative images of the area of cell coverage after a 24- to 36-hour incubation of keratinocytes with RmHV or RmAD in a cellular scratch assay. White dashed lines were digitally added to demarcate scratch borders. (B and C) Area of coverage for (B) keratinocytes and (C) fibroblasts stimulated with RmHV or RmAD in a 96-well plate. Each experiment was performed with one cell line in replicates indicated by individual dots. PBS, phosphate-buffered saline. (D) Keratinocytes pre-treated for 2 hours with antibodies to the indicated receptors or isotype control antibody were stimulated with RmHV or RmAD. (E) Mean mouse ear thickness after a 10-day application of MC903 to induce dermatitis (day 10) and after treatment with diluent (sucrose) or RmHV (day 17) for wild-type mice (+) or mice deficient (−) in TNFR1, TNFR2, or both TNF receptors. (F) Representative images of mouse ear sections at day 17 after treatment with sucrose diluent control or RmHV stained with hematoxylin and eosin (H&E). Black arrowheads indicate serocellular crusting (inflammatory cells within the layers of thick keratin). Black asterisk indicates inflammatory expansion and infiltration of the underlying dermal stroma. Scale bars, 100 μm. (G) Mean mouse ear thickness after application of MC903 and induction of dermatitis (day 10) and after treatment with sucrose diluent control or RmHV (day 17) for wild-type mice (+) or mice deficient (−) in CXCR2. Data are representative of two (E to G) or three or more (A to D) independent experiments and displayed as the means ± SEM. NS, not significant. *P < 0.05, **P < 0.01, and ***P < 0.001 determined by paired ANOVA with Dunnett correction.