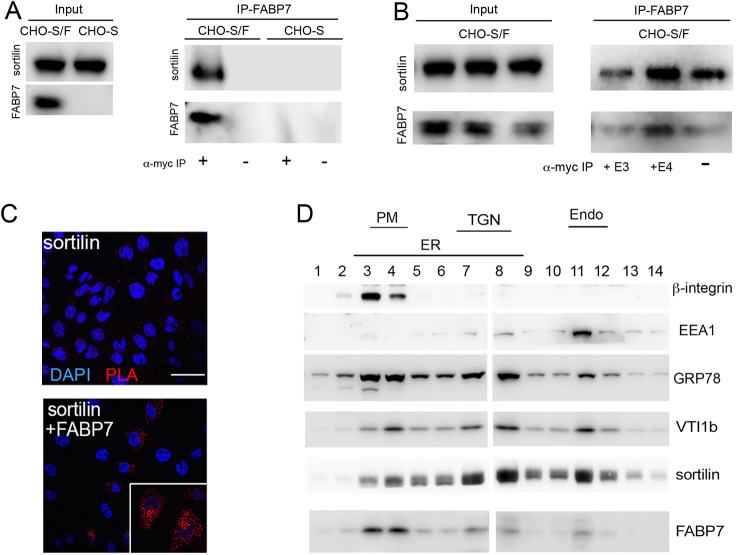
Fig. 2.
Sortilin and FABP7 interact in cells. (A) Chinese hamster ovary (CHO) cells stably overexpressing sortilin (CHO-S) or sortilin and FABP7 tagged with a Myc epitope (CHO-S/F) were used for co-immunoprecipitation (IP) experiments. Expression of sortilin and FABP7–Myc in total cell lysates of both cell lines is shown in the panel Input (15 μg of total cell lysate). In panel IP-FABP7, co-immunoprecipitation of sortilin with anti-Myc affinity resin (+) is seen in lysates from CHO-S/F but not from CHO-S cells. No immunoprecipitation of sortilin with anti-Myc affinity resin is seen in CHO-S cells, or in CHO-S/F in the absence of the affinity resin (−). (B) Co-immunoprecipitation of sortilin with FABP7-myc from CHO-S/F cells as described in A. Prior to immunoprecipitation with anti-Myc affinity resin, cells were treated with conditioned medium from HEK293 cells containing 5 µg/ml of apoE3 (+E3) or apoE4 (+E4; see Materials and Methods for details) or blank medium (−) for 24 h. (C) Proximity ligation assay (PLA) to assess close spatial proximity of sortilin and FABP7 in CHO cells. Primary antibodies were directed against sortilin or the Myc epitope in FABP7–Myc. Close proximity is detected in CHO cells expressing both sortilin and FABP7 (red signal). No PLA signal is seen in cells expressing sortilin only. Cell nuclei were counterstained with DAPI (blue). The inset shows a higher magnification image of cells positive for PLA signals for sortilin and FABP7–Myc. Scale bar: 50 µm. (D) Subcellular fractionation of CHO-S/F cells using gradient ultracentrifugation. Fractions were identified based on markers for endoplasmic reticulum (ER; GRP78), plasma membrane (PM; β-integrin), trans-Golgi network (TGN; VTI1b), and early endosomes (Endo; EEA1). FABP7 colocalizes with sortilin to the PM (fractions 3–4), TGN (fractions 7–8), and endosomes (fraction 11). Images shown are representative of results from three independent experiments.