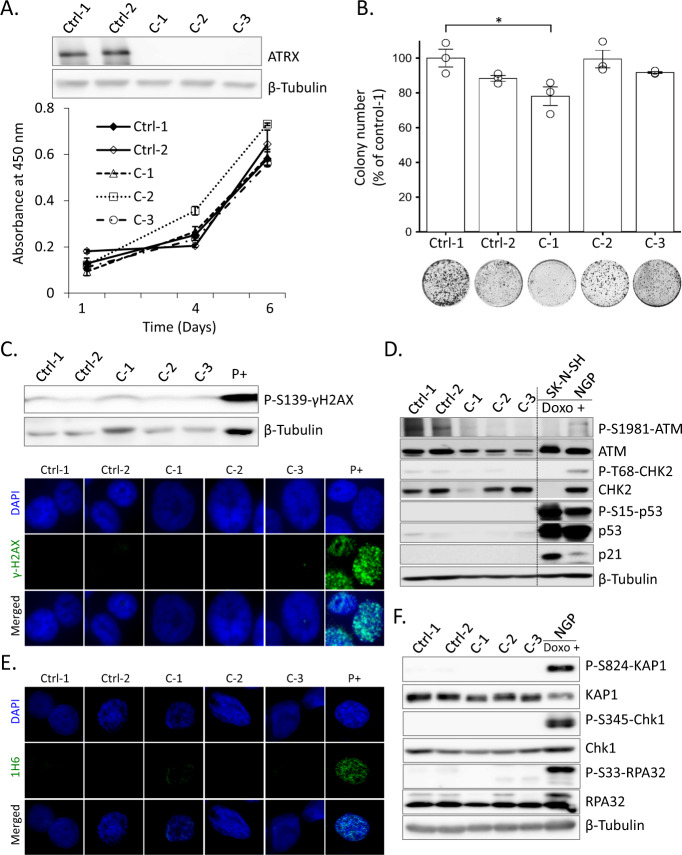
Fig. 4. ATRX deficiency-related DDRs and RS were not induced in TP53-truncated (C terminus) SK-N-AS cells.
A Western blot to confirm ATRX ablation in Cas9 control (Ctrl) and ATRX KO (C-1, C-2, and C-3) SK-N-AS cells. Growth curves show that cell viability was unchanged between Ctrl and ATRX KO SK-N-AS cells. Data are expressed as means ± standard deviation (SD), N = 3. B Colony formation assays were performed to assess the proliferative abilities of Ctrl and ATRX KO SK-N-AS cells. Lower panel, representative images for clonogenic formation. Error bars represent SD from three technical replicates. *p < 0.05; A one-way ANOVA with Dunnett’s and Tukey’s test were used for statistical analyses. C Immunoblot and IF analyses of γH2AX in ATRX-intact (Ctrl) and ATRX KO SK-N-AS cells with a positive control (parental SK-N-AS cells treated with doxorubicin, 0.5 μg/mL for 24 h). D Immunoblots for the expression of p53-ATM checkpoint proteins in cell lysates prepared from Ctrl and ATRX KO SK-N-AS cells. Doxorubicin (0.5 μg/mL)-treated two NB cell lines (SK-N-SH and NGP) were used as a positive control. E IF staining of G4 (1H6) revealed that ATRX deficiency did not cause G4 formation in ATRX KO SK-N-AS cells. The DNA G-quadruplex stabilizer CX-5461 (50 nM, 24 h) was used as a positive control to stain G4 (1H6). F Whole-cell extracts from Ctrl and ATRX KO SK-N-AS cells were analyzed by western blotting with the p-KAP1 (Ser-824), KAP1, p-Chk1 (Ser-345), Chk1, p-RPA32 (Ser-33), and RPA32 antibodies. β-Tubulin was used as a loading control. Doxorubicin (0.5 μg/mL, 24 h)-treated NGP cells were used as a positive control.