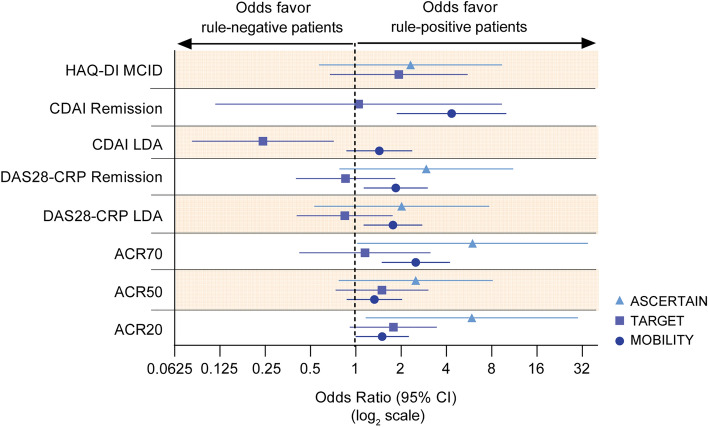
Fig. 3.
Odds ratios of achieving clinical response at week 24 in placebo- (MOBILITY, TARGET) or active-controlled studies (ASCERTAIN): rule-positive versus rule-negative patients. The patient stratification rule was the combined presence of anti-CCP and CRP > 12.3 mg/l. Data presented for MOBILITY and TARGET are placebo-adjusted. ACR20 ACR 20%, ACR50 ACR 50%, ACR70 ACR 70%, DAS28-CRP 28-joint Disease Activity Score using C-reactive protein, HAQ-DI Health Assessment Questionnaire-Disability Index, LDA low disease activity, MCID minimal clinically important difference