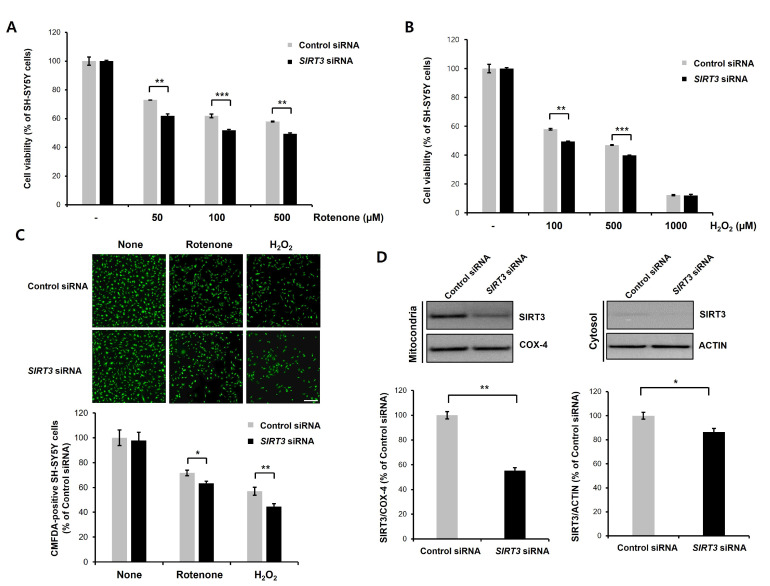
Fig. 3.
Knockdown of SIRT3 enhances rotenone- or H2O2-induced neurotoxicity in differentiated SH-SY5Y cells. (A, B) Differentiated SH-SY5Y cells were transfected with control siRNA (100 nM) or human SIRT3-specific siRNA (100 nM) for 2 days, and then a CCK-8 assay and immunoblotting were performed. Control or SIRT3 knockdown cells were treated with rotenone (50, 100, or 500 µM; A)/H2O2 (100, 500, or 1000 µM; B) for 24 h, and then CCK-8 analysis was performed. SIRT3 knockdown cells exhibited significantly increased rotenone- or H2O2-induced neuronal toxicity. Data are presented as the mean±SD of 3 independent experiments. **p<0.005; ***p<0.001 (unpaired Student’s t-test). (C) CMFDA staining of rotenone (100 µM) or H2O2 (500 µM)-treated control siRNA- or SIRT3-downregulating cells at 24 h. Then, CMFDA-positive neurons were counted under a fluorescence microscope. Data are presented as the mean±SD of 3. *p<0.05; **p<0.005 (unpaired Student’s t-test). Scale bars, 200 µm. (D) Protein levels of mitochondrial and cytosolic SIRT3 in control and SIRT3 knockdown cells. SIRT3 protein levels were greatly decreased in the mitochondrial fraction of SIRT3 knockdown cells. COX-IV (mitochondrial fraction) and actin (cytosolic fraction) were used for normalization. Data are presented as the mean±SD of 3 independent experiments. *p<0.05; **p<0.005 (unpaired Student’s t-test).