Abstract
Background
Intravenous immunoglobulin (IVIG) has been used as an immunomodulatory therapy to counteract severe systemic inflammation in coronavirus disease 2019 (COVID-19). But its use in COVID-19 related acute respiratory distress syndrome (ARDS) is not well established.
Methods
We conducted a retrospective analysis of electronic health records of COVID-19 patients admitted to intensive care units (ICUs) at Hazm Mebaireek General Hospital, Qatar, between March 7, 2020 and September 9, 2020. Patients receiving invasive mechanical ventilation for moderate-to-severe ARDS were divided into two groups based on whether they received IVIG therapy or not. The primary outcome was all-cause ICU mortality. Secondary outcomes studied were ventilator-free days and ICU-free days at day-28, and incidence of acute kidney injury (AKI). Propensity score matching was used to adjust for confounders, and the primary outcome was compared using competing-risks survival analysis.
Results
Among 590 patients included in the study, 400 received routine care, and 190 received IVIG therapy in addition to routine care. One hundred eighteen pairs were created after propensity score matching with no statistically significant differences between the groups. Overall ICU mortality in the study population was 27.1%, and in the matched cohort, it was 25.8%. Mortality was higher among IVIG-treated patients (36.4% vs. 15.3%; sHR 3.5; 95% CI 1.98–6.19; P < 0.001). Ventilator-free days and ICU-free days at day-28 were lower (P < 0.001 for both), and incidence of AKI was significantly higher (85.6% vs. 67.8%; P = 0.001) in the IVIG group.
Conclusion
IVIG therapy in mechanically ventilated patients with COVID-19 related moderate-to-severe ARDS was associated with higher ICU mortality. A randomized clinical trial is needed to confirm this observation further.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12890-021-01717-x.
Keywords: Acute respiratory distress syndrome, COVID-19, ICU mortality, Intravenous immunoglobulin, Mechanical ventilation
Background
Coronavirus disease 2019 (COVID-19) is a highly infectious acute respiratory disease caused by a novel coronavirus, subsequently named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) [1]. The first human cases of COVID-19 were reported in Wuhan City, China, in December 2019. Since then, the disease has rapidly spread worldwide, with World Health Organization (WHO) formally declaring it a pandemic on March 11, 2020 [2].
Over 4.8 million deaths have been reported worldwide due to COVID-19 [3]. The leading cause of death is respiratory failure due to acute respiratory distress syndrome (ARDS). In addition, almost half of the patients with COVID-19 receiving invasive mechanical ventilation died, based on the case fatality rates reported in a recent meta-analysis [4]. There is increasing evidence that a hyperinflammatory response to SARS-CoV-2 contributes to disease severity and death in COVID-19. Patients with severe disease have increased serum levels of proinflammatory cytokines such as interleukin (IL)-1, IL-2, IL-6, tumor necrosis factor (TNF)-α, and interferon (IFN)-γ [5, 6]. Therefore, an effective therapy that modulates inflammatory response, prevents clinical deterioration and improves mortality is urgently needed.
Intravenous immunoglobulin (IVIG) is a blood product prepared from the serum pooled from thousands of healthy donors. The main component of IVIG is the serum IgG fraction with traces of IgA and IgM. IVIG exerts an immunomodulatory action, involving both innate (phagocytic leukocytes, natural killer cells, and cytokines) and adaptive (B cells, T cells, and antibodies) immunity [7]. It has been successfully used to treat dermatomyositis, Guillain–Barre syndrome, immune cytopenia, post-bone marrow transplantation, vasculitis, and Kawasaki disease [8]. Due to its anti-inflammatory effect, IVIG may suppress the hyperactive immune response associated with severe COVID-19 pneumonia and improve clinical outcomes. However, only a few studies with inconsistent results have investigated the use of IVIG in critically ill SARS-CoV-2 infected patients [9, 10]. Moreover, none of the available literature reports outcomes of IVIG therapy in COVID-19 related ARDS. This prompted us to conduct a retrospective study to evaluate the efficacy and safety of IVIG in COVID-19 pneumonia patients requiring invasive mechanical ventilation for moderate-to-severe ARDS.
Methods
Study population, design, and setting
We retrospectively analyzed electronic health record data of patients admitted to ICUs at Hazm Mebaireek General Hospital, Qatar, between March 7, 2020 and September 9, 2020. The Medical Research Center (MRC) at Hamad Medical Corporation, Qatar, approved this study and waived the requirement for informed consent (protocol ID MRC-01-20-853). Inclusion criteria were: COVID-19 positivity as determined by reverse-transcription polymerase chain reaction (RT-PCR) of nasopharyngeal swabs, age above 18 years, respiratory failure requiring invasive mechanical ventilation, and moderate-to-severe ARDS (PaO2/FiO2 ≤ 200 mm Hg) as defined by the Berlin criteria [11]. Patients who received IVIG for indications other than COVID-19 related ARDS or had cardiac arrest before ICU admission were excluded. The study population was divided into two groups based on receiving routine care or IVIG plus routine care during their ICU stay. All patients received steroid and antiviral therapy as per the hospital's policy for COVID-19 management unless contraindicated. Prophylactic-, intermediate- or therapeutic-dose of anticoagulation was administered based on the patient's risk stratification as low-, intermediate- or high-risk for thromboembolic disease (anticoagulation protocol in Additional file 1: Fig. S1). IVIG was given to patients at the treating physician's discretion if there was a persistent increase in oxygen requirement and worsening laboratory parameters (C-reactive protein and serum ferritin level), suggestive of disease progression. The IVIG treatment group received a minimum one dose of 0.4 g/kg of IVIG. Further doses of IVIG were given on consecutive days, to a maximum of 5 doses, based on the treating physician's decision. Patients were closely monitored for immediate adverse effects like skin rash, arrhythmias, hypotension, and anaphylaxis.
Data collection
Baseline data were collected at the time of ICU admission. Information collected were demographic characteristics, comorbid conditions, blood test results, PaO2/FiO2 ratio, vasopressor use, sequential organ failure assessment (SOFA) score, immune-modulating, and antiviral drugs received.
Outcomes
The primary outcome studied was all-cause ICU mortality. Secondary outcomes included ventilator-free days and ICU-free days at day-28, and incidence of acute kidney injury (AKI) defined by the Kidney Disease Improving Global Outcomes (KDIGO) criteria as an increase in serum creatinine level by ≥ 0.3 mg/dl (≥ 26.5 μmol/L) within 48 h or increase in serum creatinine to ≥ 1.5 times baseline [12]. Sub-group analysis was done for patients based on PaO2/FiO2 ratio, serum ferritin level, time from ICU admission to IVIG therapy, and doses of IVIG received.
Statistical analysis
We used propensity score matching to account for the non-random treatment allocation and to adjust for confounders. Propensity scores were generated based on age, sex, body mass index (BMI), comorbidities, SOFA score, PaO2/FiO2 ratio, baseline laboratory results, and other drug therapies. The propensity score overlap was assessed graphically. One-to-one matching with a caliper width of 0.2 of the standard deviation of the logit of the propensity score and no replacement was used to select a matching control group [13]. The post-matching covariate balance was assessed using the standardized differences. The primary outcome was compared using survival analysis. We included the treatment with IVIG as a discrete time-dependent covariate to minimize the risk of immortal time bias. Because the discharge from ICU does not fulfill the assumption of non-informative censoring, Fine-and-Gray's competing risk model was used for the primary outcome with ICU discharge as a competing event. The treatment effect on the primary outcome was described as a sub-distribution hazard ratio (sHR) with a 95% confidence interval. A robust variance estimator was used to account for correlations resulting from matching. Multiple imputation procedure was used to deal with missing data (BMI, seven values; D-dimer, nine values; and ferritin, three values). P values of less than 0.05 were considered statistically significant. All statistical analyses were conducted using Stata/MP 16.0 for Windows.
Results
Characteristics of patients
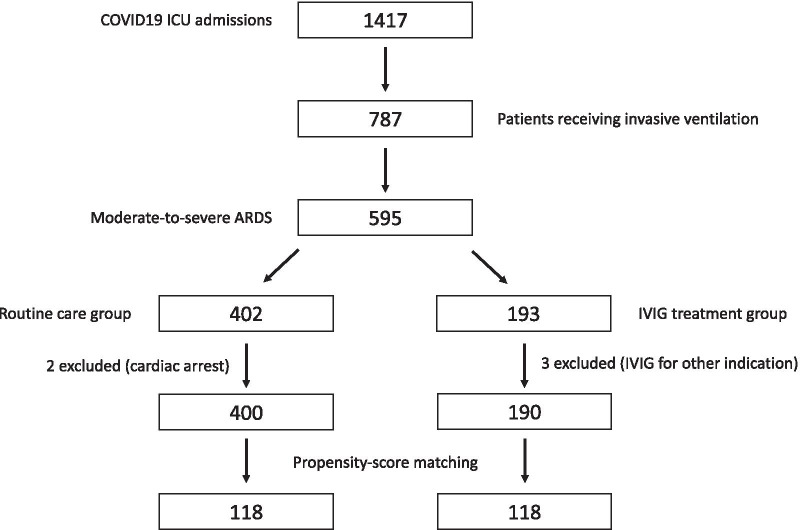
During the study period (between March 7, 2020 and September 9, 2020), 1417 patients were admitted to ICUs at Hazm Mebaireek General Hospital with the diagnosis of COVID-19. Seven hundred eighty-seven patients received invasive mechanical ventilation, of which 595 (75.6%) had moderate-to-severe ARDS. We excluded three patients who received IVIG for other indications from the treated group and two patients admitted to the ICU post-cardiac arrest from the control group. The remaining cohort of 590 patients comprised 190 treated with IVIG and 400 in the routine care group (Fig. 1).
Fig. 1.
Flow chart showing selection of patients. COVID19, corona virus disease 2019; ICU intensive care unit; ARDS, acute respiratory distress syndrome; IVIG, intraveneous immunoglobulin
A comparison between unmatched and propensity score-matched groups is shown in Table 1. Before matching, the patients in the IVIG group were older, had a higher prevalence of hypertension, dyslipidemia, and hemodialysis, had lower PaO2/FiO2 ratio, lesser vasopressor use, lower SOFA score, raised alanine transaminase (ALT), more use of dexamethasone and lesser use of methylprednisolone, hydrocortisone, tocilizumab, lopinavir-ritonavir, and oseltamivir. In the IVIG group, the median time from ICU admission to initiation of IVIG therapy was 6.28 days (IQR 2.1–11.9 days). The median cumulative dose of IVIG received was 150 g (IQR 105–235 g), and the median number of doses received was 4 (IQR 3–5).
Table 1.
Patient characteristics
| Variable | Total (n = 590) | Unmatched | Matched | ||||
|---|---|---|---|---|---|---|---|
| Routine care (n = 400) | IVIG (n = 190) | P value | Routine care (n = 118) | IVIG (n = 118) | P value | ||
| Age (years) | 53 (42–62) | 51 (41.5–61) | 56.5 (47–65) | < 0.001 | 52 (42–60) | 53.5 (44–60) | 0.36 |
| Gender | |||||||
| Male | 555 (94.1%) | 377 (94.2%) | 178 (93.7%) | 0.79 | 112 (94.9%) | 112 (94.9%) | 1.00 |
| Female | 35 (5.9%) | 23 (5.8%) | 12 (6.3) | 6 (5.1%) | 6 (5.1%) | ||
| BMI (kg/m2) | 27.2 (24.2–30.4) | 26.9 (24.2–30.7) | 27.6 (24.7–30.1) | 0.50 | 26.7 (23.9–30.1) | 27.69 (24.7–29.4) | 0.54 |
| Co-morbidities | |||||||
| Diabetes mellitus | 288 (48.8%) | 198 (49.5%) | 90 (47.4%) | 0.63 | 51 (43.2%) | 55 (46.6%) | 0.60 |
| Hypertension | 290 (49.2%) | 184 (46.0%) | 106 (55.8%) | 0.03 | 59 (50.0%) | 58 (49.2%) | 0.9 |
| Dyslipidemia | 68 (11.5%) | 38 (9.5%) | 30 (15.8%) | 0.03 | 10 (8.5%) | 12 (10.2%) | 0.65 |
| Coronary artery disease | 77 (13.1%) | 45 (11.3%) | 32 (16.8%) | 0.06 | 12 (10.2%) | 14 (11.9%) | 0.68 |
| Chronic kidney disease | 58 (9.8%) | 44 (11.0%) | 14 (7.4%) | 0.17 | 4 (3.4%) | 5 (4.2%) | 1.00 |
| Hemodialysis | 33 (5.6%) | 0 (0%) | 33 (17.4%) | < 0.001 | 0 (0%) | 0 (0%) | |
| Chronic respiratory illness | 40 (6.8%) | 22 (5.5%) | 18 (9.5%) | 0.07 | 13 (11%) | 8 (6.8%) | 0.25 |
| Chronic liver disease | 11 (1.9%) | 10 (2.5%) | 1 (0.5%) | 0.12 | 1 (0.8%) | 1 (0.8%) | 1.00 |
| Malignancy | 19 (3.2%) | 11 (2.8%) | 8 (4.2%) | 0.35 | 7 (5.9%) | 5 (4.2%) | 0.55 |
| PaO2/FiO2 ratio (mm Hg) | 124 (92–141) | 130 (97.5–150) | 105.35 (80–134) | < 0.001 | 123 (81–140) | 110.7 (87.1–136) | 0.38 |
| Vasopressor | 415 (70.3%) | 303 (75.8%) | 96 (50.5%) | < 0.001 | 71 (60.2%) | 67 (56.8%) | 0.60 |
| Sofa score | 2 (2–5) | 3 (2–5) | 2 (1–4) | < 0.001 | 2 (2–4) | 2 (1–4) | 0.06 |
| Laboratory data | |||||||
| CRP (mg/L) | 163.3 (91.9–246.7) | 166.5 (99.6–245.3) | 156.2 (69.5–255) | 0.29 | 175.4 (90.9–257) | 152 (72–232.1) | 0.16 |
| Ferritin (mcg/L) | 1057 (637–1625) | 1017.5 (638.5–1559) | 1118 (638–1942) | 0.14 | 1168.5 (693–1565) | 1118.5 (688–1924) | 0.27 |
| D-Dimer (mcg/mL) | 1.25 (0.68–3.95) | 1.35 (0.68–4.23) | 1.17 (0.74–3.24) | 0.60 | 1.43 (0.61–4.6) | 1.13 (0.74–2.88) | 0.99 |
| Platelets (109/L) | 233.5 (184–306) | 240 (188–312) | 221 (176–293) | 0.06 | 249.5 (195–317) | 236 (186–308) | 0.51 |
| Creatinine (micromol/L) | 85 (70–111) | 84 (69–110) | 93 (71–113) | 0.08 | 78 (64–98) | 86 (68–108) | 0.07 |
| Bilirubin (micromol/L) | 11 (8–15) | 11 (8–15) | 11 (7–16) | 0.74 | 10 (8–14) | 10.25 (7–14.5) | 0.95 |
| ALT (IU/L) | 40 (25–64) | 38 (24–59) | 45 (26–70) | 0.03 | 37 (23–64) | 45 (28–70) | 0.053 |
| AST (IU/L) | 51 (35–80) | 50 (35–77) | 54 (35–91) | 0.07 | 50.5 (36–86) | 54.5 (36–91) | 0.42 |
| Corticosteroids | |||||||
| Dexamethasone | 168 (28.5%) | 96 (24.0%) | 72 (37.9%) | < 0.001 | 32 (27.1%) | 41 (34.7%) | 0.2 |
| Methylprednisolone | 446 (75.6%) | 331 (82.8%) | 115 (60.5%) | < 0.001 | 88 (74.6%) | 79 (66.9%) | 0.2 |
| Hydrocortisone | 142 (24.1%) | 110 (27.5%) | 32 (16.8%) | 0.005 | 15 (12.7%) | 14 (11.9%) | 0.84 |
| Tocilizumab | 329 (55.8%) | 251 (62.7%) | 78 (41.1%) | < 0.001 | 56 (47.5%) | 54 (45.8%) | 0.79 |
| Interferon | 65 (11.0%) | 49 (12.3%) | 15(7.9%) | 0.12 | 11 (9.3%) | 10 (8.5%) | 0.82 |
| Anti-viral drugs | |||||||
| Favipiravir | 78 (13.2%) | 47 (11.8%) | 31 (16.3%) | 0.13 | 17 (14.4%) | 23 (19.5%) | 0.30 |
| Lopinavir-Ritonavir | 306 (51.9%) | 229 (57.3%) | 77 (40.5%) | < 0.001 | 58 (49.2%) | 51 (43.2%) | 0.36 |
| Oseltamivir | 355 (60.2%) | 267 (66.8%) | 88 (46.3%) | < 0.001 | 63 (53.4%) | 58 (49.2%) | 0.51 |
| Remdesivir | 2 (0.3%) | 0 (0%) | 2 (1.1%) | 0.10 | 0 (0%) | 0 (0%) | |
| Ribavirin | 61 (10.3%) | 50 (12.5%) | 11 (5.8%) | 0.01 | 9 (7.6%) | 7 (5.9%) | 0.60 |
Data are presented as count (%) or median (interquartile range) unless otherwise indicated
Laboratory data and SOFA score were obtained at baseline on ICU admission
BMI, body mass index; PaO2/FiO2 ratio, ratio of partial pressure arterial oxygen and fraction of inspired oxygen; SOFA score, sequential organ failure assessment score; CRP, C-reactive protein; ALT, alanine transaminase; AST, aspartate aminotransferase
Propensity score matching generated 118 matched sets. Post matching, the two groups did not have any statistically significant differences (Table 1). In the matched cohort, the median time from ICU admission to initiation of IVIG therapy was 6 days (IQR 2.2–11.1 days). The median cumulative dose of IVIG received was 152.0 g (IQR 108.0–235.0 g), and the median number of doses received was 5.0 (IQR 3.0–5.0).
Outcomes
The all-cause ICU mortality for COVID-19 pneumonia patients admitted with respiratory failure requiring invasive mechanical ventilation for moderate-severe ARDS was 27.1%, and for the matched cohort, it was 25.8%. ICU mortality was significantly higher in the IVIG group (36.4% vs. 15.3% for IVIG and routine care, respectively; sHR 3.5; 95% CI 1.98–6.19; P < 0.001). In the propensity score-matched and propensity score-adjusted survival analysis, there was an increased risk of death in the IVIG group compared to the routine care group (Table 2). Results were similar, with an increased risk of death in the IVIG-treated patients for the subgroups based on PaO2/FiO2 ratio and serum ferritin level. Association of IVIG with increased ICU mortality was significant regardless of the time from ICU admission to IVIG therapy and the number of doses received (Table 3).
Table 2.
Association of IVIG treatment with mortality
| Total (n) | sHR (95% confidence interval) | P value | |
|---|---|---|---|
| PS matched analysis | 236 | 3.50 (1.98–6.19) | < 0.001 |
| PS adjusted analysis | 556* | 2.98 (1.92–4.60) | < 0.001 |
IVIG, intravenous immunoglobulin; PS, propensity score; sHR, sub-distribution hazard ratio
*Propensity score could not be calculated for 34 patients due to complete separation by baseline characteristics
Table 3.
Association of IVIG treatment with mortality among sub-groups
| Sub-groups | sHR (95% confidence interval) | P value |
|---|---|---|
| PaO2/FiO2 ratio | ||
| > 100 mm Hg | 3.44 (2.04–5.78) | < 0.001 |
| ≤ 100 mm Hg | 2.17 (1.07–4.41) | 0.03 |
| Serum ferritin level | ||
| < 1000 mcg/L | 3.45 (1.87–6.38) | < 0.001 |
| ≥ 1000 mcg/L | 2.79 (1.52–5.11) | 0.001 |
| Time from ICU admission to IVIG therapy | ||
| < 5 days | 2.65 (1.07–6.56) | 0.035 |
| ≥ 5 days | 3.89 (1.85–8.18) | < 0.001 |
| Number of IVIG doses received | ||
| ≤ 3 doses | 3.72 (1.39–9.91) | 0.009 |
| > 3 doses | 3.42 (1.69–6.93) | 0.001 |
IVIG, intravenous immunoglobulin; sHR, sub-distribution hazard ratio; PaO2/FiO2 ratio, ratio of partial pressure arterial oxygen and fraction of inspired oxygen; mcg/L, microgram per liter
Compared to routine care group, ventilator-free days at day-28 were lower in the IVIG group [median (IQR); 0 (0–18) vs. 22 (7–24) days; P < 0.001], and ICU-free days at day-28 were also lower in the IVIG group [median (IQR); 0 (0–8.8) vs. 16 (0–20) days; P < 0.001]. Additionally, the incidence of AKI was significantly higher in the IVIG group (85.6% vs. 67.8% for IVIG and routine care, respectively; P = 0.001).
Discussion
Our single-center retrospective study revealed a significant association of IVIG therapy with higher ICU mortality in patients with COVID-19 pneumonia receiving invasive mechanical ventilation for moderate-to-severe ARDS. We confirmed these results after propensity score matching to account for the differences between the two groups.
Only a few randomized controlled studies have been conducted to evaluate the efficacy of IVIG therapy in COVID-19 pneumonia. Gharebaghi et al. have reported that administration of IVIG to 30 patients with severe COVID-19 infection who did not respond to initial treatment significantly reduced the in-hospital mortality (20.0% in the treatment group vs. 48.3% in the control group; P = 0.025). However, the authors did not report the severity of ARDS and the percentage of patients receiving invasive mechanical ventilation [14]. Another pilot randomized controlled trial showed that IVIG 0.5 g/kg daily for three days with concomitant methylprednisolone 40 mg significantly improved hypoxia and reduced progression to mechanical ventilation in COVID19 patients [15]. The clinical improvement found in this study cannot be generalized because of the small sample size and concomitant use of methylprednisolone therapy that may have confounded the results. Recently, Tabarsi et al. demonstrated that IVIG in combination with hydroxychloroquine and lopinavir/ritonavir for SARS-CoV-2 patients did not reduce mortality or need for mechanical ventilation, and did not improve radiological findings. However, the potential benefit of IVIG monotherapy couldn't be evaluated in this study due to the use of combination therapy with hydroxychloroquine and lopinavir/ritonavir [16].
The novelty of our work comes from exclusively studying critically ill COVID-19 pneumonia patients receiving invasive mechanical ventilation for moderate-to-severe ARDS. Based on a global literature survey, the mortality rate in COVID-19 associated ARDS was 45%, and the incidence of ARDS among non-survivors of COVID-19 was 90%. In the same study, the mortality rate of patients who received invasive mechanical ventilation was 59% [17]. Overall ICU mortality of our patients with moderate-to-severe ARDS receiving invasive mechanical ventilation was lower (27.1%). This could be attributed to the younger age of our population (median age 53 years, Table 1) and is consistent with previous reports of higher mortality with increasing age [18].
The higher mortality observed in our patients who received IVIG could be related to multiple factors. Firstly, we observed a higher incidence of AKI in the IVIG group, and based on a recent report, the occurrence of AKI had increased the risk of death by 60% in patients with COVID-19 [19]. Secondly, IVIG was administered after ICU admission and receiving invasive mechanical ventilation for moderate-to-severe ARDS, which is late in the course of the disease. The median time from ICU admission to IVIG therapy was 6.28 days in our study. Xie et al. has reported that IVIG therapy for COVID-19 pneumonia within 48 h of ICU admission improved clinical outcomes [20]. Thirdly, thromboembolic events are high in SARS-CoV-2 infected individuals with significantly increased odds of mortality [21]. Also, previous studies have suggested an association between IVIG and increased risk of thromboembolic events [22, 23]. Hence, initiating IVIG therapy in patients with predictive factors for thrombotic complications could have resulted in worse clinical outcomes in our cohort. Lastly, though the control and treatment groups were matched based on their baseline characteristics (including PaO2/FiO2 ratio and SOFA score) at ICU admission, IVIG was administered mainly by physicians as salvage therapy to deteriorating patients who had not responded to initial management.
The dose and duration of IVIG administered to our patients were based on established practice in immune modulation therapy for other diseases [24], as there are no standardized guidelines for its use in COVID-19 patients. In a retrospective case series of 12 patients where IVIG appeared to improve the clinical course, the total dose administered ranged from 0.5 to 2.0 g/kg (median 1.25 g/kg) distributed over 1–4 daily doses [25]. Further studies are required to investigate the dose, duration, and appropriate time for initiation of IVIG therapy which might benefit COVID-19 patients without having adverse effects.
Our study has few limitations. We used propensity score matching to adjust for known confounders, but due to the retrospective design, we could not entirely exclude the possibility of unmeasured confounding factors that might have led to worse outcomes in the IVIG group. The study was conducted in a single center in Qatar, thereby limiting the generalization of these results to other institutions. Our hospital was a tertiary-care referral center for COVID-19 in the State of Qatar, receiving patients from numerous other healthcare facilities. Despite being rigorous in our data collection and analysis approach, there were missing data for some variables that could have affected the outcomes. We did not define the phenotype of our patients as hypo- or hyper-inflammatory based on the severity of systemic inflammation [26] and hence could not study the role of IVIG in modulating the immune response in hyper-inflammatory COVID-19 ARDS.
Conclusions
The results of our single-center retrospective study revealed a higher ICU mortality rate, lesser ventilator-free days and ICU-free days at day-28, and higher incidence of AKI in mechanically ventilated patients who received IVIG therapy for COVID-19 related moderate-to-severe ARDS. Despite the limitations, this study highlights the possibility of unfavorable outcomes with IVIG therapy in SARS-CoV-2 infected patients. A multicenter, randomized clinical trial is warranted to investigate further IVIG's efficacy and safety in critically ill COVID-19 patients.
Supplementary Information
Additional file 1. Anticoagulation protocol for critically ill COVID-19 patients at Hazm Mebaireek General Hospital, Qatar.
Acknowledgements
Not applicable.
Abbreviations
- AKI
Acute kidney injury
- ARDS
Acute respiratory distress syndrome
- COVID-19
Coronavirus disease 2019
- CRP
C-reactive protein
- ICU
Intensive care unit
- IVIG
Intravenous immunoglobulin
- PaO2/FiO2
Ratio of partial pressure arterial oxygen and the fraction of inspired oxygen
- SARS-CoV-2
Severe acute respiratory syndrome coronavirus 2
- SOFA
Sequential organ failure assessment
Authors' contributions
HSA, the principal investigator, had complete access to data and contributed to the study design, result interpretation, and writing of the manuscript. MAW and AA conducted electronic health record review and data collection. MOS and HAM performed data analysis, literature review and contributed to the study design and writing of the manuscript. MSE, NS, DCA, MAA, SG, and MYK contributed to the literature review, study design, drafting, and manuscript editing. AJN and ASM contributed to the conceptualization, data analysis, and editing of the manuscript. All authors read and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of data and materials
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The Medical Research Center (MRC) at Hamad Medical Corporation, Qatar, approved this study and waived the requirement for informed consent (protocol ID MRC-01-20-853).
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Naming the coronavirus disease (COVID-19) and the virus that causes it. [Online]. Available: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. Accessed 22 Apr 2021.
- 2.Cucinotta D, Vanelli M. WHO declares COVID-19 a pandemic. Acta Biomed. 2020;91(1):157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.WHO Coronavirus (COVID-19) dashboard. [Online]. Available: https://covid19.who.int. Accessed 17 Oct 2021.
- 4.Lim ZJ, Subramaniam A, Ponnapa Reddy M, Blecher G, Kadam U, Afroz A, Billah B, Ashwin S, Kubicki M, Bilotta F, Curtis JR, Rubulotta F. Case fatality rates for patients with COVID-19 requiring invasive mechanical ventilation. A meta-analysis. Am J Respir Crit Care Med. 2021;203(1):54–66. doi: 10.1164/rccm.202006-2405OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mehta P, McAuley DF, Brown M, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Del Valle DM, Kim-Schulze S, Huang HH, et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat Med. 2020;26:1636–1643. doi: 10.1038/s41591-020-1051-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwab I, Nimmerjahn F. Intravenous immunoglobulin therapy: how does IgG modulate the immune system? Nat Rev Immunol. 2013;13(3):176–189. doi: 10.1038/nri3401. [DOI] [PubMed] [Google Scholar]
- 8.Jolles S, Sewell WA, Misbah SA. Clinical uses of intravenous immunoglobulin. Clin Exp Immunol. 2005;142(1):1–11. doi: 10.1111/j.1365-2249.2005.02834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shao Z, Feng Y, Zhong L, Xie Q, Lei M, Liu Z, Wang C, Ji J, Liu H, Gu Z, Hu Z, Su L, Wu M, Liu Z. Clinical efficacy of intravenous immunoglobulin therapy in critical ill patients with COVID-19: a multicenter retrospective cohort study. Clin Transl Immunol. 2020;9(10):e1192. doi: 10.1002/cti2.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Yang Y, Yang N, et al. Effectiveness of intravenous immunoglobulin for children with severe COVID-19: a rapid review. Ann Transl Med. 2020;8(10):625. doi: 10.21037/atm-20-3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 12.Clinical Practice Guidelines for Acute Kidney Injury 2012. [Online]. Available: https://kdigo.org/guidelines/acute-kidney-injury/. Accessed 22 Apr 2021.
- 13.Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–161. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gharebaghi N, Nejadrahim R, Mousavi SJ, et al. The use of intravenous immunoglobulin gamma for the treatment of severe coronavirus disease 2019: a randomized placebo-controlled double-blind clinical trial. BMC Infect Dis. 2020;20:786. doi: 10.1186/s12879-020-05507-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakoulas G, Geriak M, Kullar R, et al. Intravenous immunoglobulin plus methylprednisolone mitigate respiratory morbidity in coronavirus disease 2019. Crit Care Explor. 2020;2(11):e0280. doi: 10.1097/CCE.0000000000000280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabarsi P, Barati S, Jamaati H, Haseli S, Marjani M, Moniri A, Abtahian Z, Dastan A, Yousefian S, Eskandari R, Saffaei A, Monjazebi F, Vahedi A, Dastan F. Evaluating the effects of intravenous immunoglobulin (IVIg) on the management of severe COVID-19 cases: a randomized controlled trial. Int Immunopharmacol. 2021;90:107205. doi: 10.1016/j.intimp.2020.107205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tzotzos SJ, Fischer B, Fischer H, Zeitlinger M. Incidence of ARDS and outcomes in hospitalized patients with COVID-19: a global literature survey. Crit Care. 2020;24(1):516. doi: 10.1186/s13054-020-03240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yanez ND, Weiss NS, Romand JA, Treggiari MM. COVID-19 mortality risk for older men and women. BMC Public Health. 2020;20(1):1742. doi: 10.1186/s12889-020-09826-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Russo E, Esposito P, Taramasso L, et al. Kidney disease and all-cause mortality in patients with COVID-19 hospitalized in Genoa, Northern Italy. J Nephrol. 2021;34(1):173–183. doi: 10.1007/s40620-020-00875-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xie Y, Cao S, Dong H, et al. Effect of regular intravenous immunoglobulin therapy on prognosis of severe pneumonia in patients with COVID-19. J Infect. 2020;81(2):318–356. doi: 10.1016/j.jinf.2020.03.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malas MB, Naazie IN, Elsayed N, Mathlouthi A, Marmor R, Clary B. Thromboembolism risk of COVID-19 is high and associated with a higher risk of mortality: a systematic review and meta-analysis. EClin Med. 2020;29:100639. doi: 10.1016/j.eclinm.2020.100639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ammann EM, Jones MP, Link BK, et al. Intravenous immune globulin and thromboembolic adverse events in patients with hematologic malignancy. Blood. 2016;127(2):200–207. doi: 10.1182/blood-2015-05-647552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marie I, Maurey G, Hervé F, Hellot MF, Levesque H. Intravenous immunoglobulin-associated arterial and venous thrombosis; report of a series and review of the literature. Br J Dermatol. 2006;155(4):714–721. doi: 10.1111/j.1365-2133.2006.07390.x. [DOI] [PubMed] [Google Scholar]
- 24.Perez EE, Orange JS, Bonilla F, Chinen J, Chinn IK, Dorsey M, El-Gamal Y, Harville TO, Hossny E, Mazer B, Nelson R, Secord E, Jordan SC, Stiehm ER, Vo AA, Ballow M. Update on the use of immunoglobulin in human disease: a review of evidence. J Allergy Clin Immunol. 2017;139(3S):S1–S46. doi: 10.1016/j.jaci.2016.09.023. [DOI] [PubMed] [Google Scholar]
- 25.Herth FJF, Sakoulas G, Haddad F. Use of intravenous immunoglobulin (Prevagen or Octagam) for the treatment of COVID-19: retrospective case series. Respiration. 2020;99(12):1145–1153. doi: 10.1159/000511376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang J, Whebell SF, Sanderson B, Retter A, Daly K, Paul R, Barrett N, Agarwal S, Lams BE, Meadows C, Terblanche M, Camporota L. Phenotypes of severe COVID-19 ARDS receiving extracorporeal membrane oxygenation. Br J Anaesth. 2021;126(3):e130–e132. doi: 10.1016/j.bja.2020.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Anticoagulation protocol for critically ill COVID-19 patients at Hazm Mebaireek General Hospital, Qatar.
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.