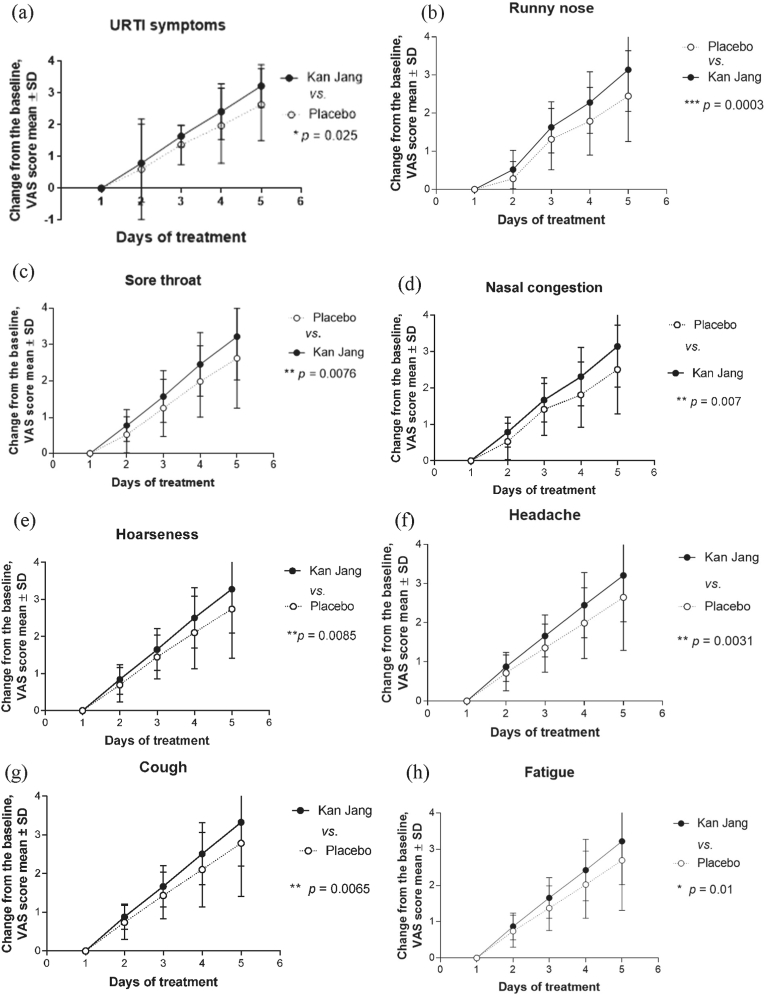
Fig. 3.
Effects of Kan Jang® (Group B) and placebo (Group A) on inflammatory symptoms, as assessed by the change in the total VAS score from baseline (mean ± SD) during the 5 days of treatment. Statistically significant (p = 0.025, two-way ANOVA) interaction effect between treatment groups and response over time showed a significant difference between Kan Jang (group B) vs. placebo (Group A). Y-axis: reduction (change from baseline) in the total symptom VAS score.