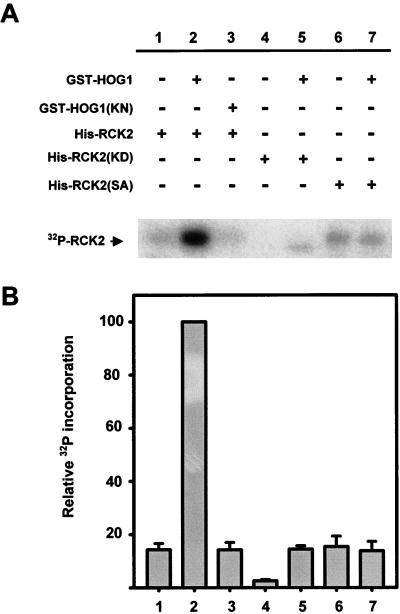
FIG. 5.
Induction of Rck2 activity by Hog1 phosphorylation. (A) Purified wild-type Rck2 protein or the indicated mutant forms of Rck2 were incubated with (+) or without (−) wild-type GST-HOG1 or the catalytically inactive mutant version GST-HOG1(KN), in the presence of kinase buffer and cold ATP. After 15 min at 30°C, HOG1 was removed by affinity chromatography and Rck2 was further incubated in the presence of kinase buffer and radioactive ATP. Phosphorylated proteins were separated by SDS-PAGE and detected by autoradiography. The position of Rck2 is indicated on the left. A representative experiment is shown. (B) The intensity of autophosphorylation was quantified with a phosphorimager (Fuji BAS1000). Samples from three independent experiments were measured, and the intensity of each band was normalized to that of lane 2. Error bars indicate standard errors of the means.