Abstract
Dysregulation of phosphorus homeostasis resulting in hypophosphatemia is common in cancer patients and can result in serious complications and impact outcomes. Several factors, including critical illness, nutritional status, cancer type and therapy, influence the development of hypophosphatemia. Hypophosphatemia can develop as a result of phosphaturic mesenchymal tumors or as a paraneoplastic phenomenon. The clinical presentation for hypophosphatemia varies depending on the duration and severity of the hypophosphatemia and affects several organ systems. Among other serious effects, hypophosphatemia can impair tissue oxygenation and can cause hemolysis, leukocyte and platelet dysfunction, encephalopathy, seizures, arrhythmias, cardiomyopathy, rhabdomyolysis and coma. Multiple studies have demonstrated that hypophosphatemia is an adverse prognostic marker in inpatients with increased in-hospital stay, mortality and postoperative complications. The phosphate level is homeostatically regulated and maintained in a narrow range by three main hormones: parathyroid hormone, fibroblast growth factor 23 and 1,25-dihydroxyvitaminD3. Together, these hormones regulate how the intestine, kidneys and bones traffic phosphorus. Several hematological malignancies and cancer therapies are associated with proximal tubular dysfunction (Fanconi syndrome), resulting in phosphaturia. Caution should be taken with parenteral administration of phosphate salts, because secondary complications can develop, principally due to hypocalcemia. The general approach to hypophosphatemia should target the underlying cause. Early recognition and prevention are essential and the approach to hypophosphatemia in the cancer patient, because of the nuances and complexity, should be multidisciplinary.
Keywords: electrolytes, Fanconi syndrome, hypophosphatemia, onconephrology, phosphate, phosphorus, phosphaturia
INTRODUCTION
Hypophosphatemia, defined as serum phosphorus ˂2.5 mg/dL, is a common occurrence in cancer patients and is associated with increased morbidity and mortality. Phosphorus is essential for the normal physiologic function of all cells and its homeostasis is frequently interrupted by cancer and cancer therapy. In the present article we review the epidemiology, clinical outcomes, physiology, pathophysiology, etiology and treatment of hypophosphatemia in the context of malignancy. Note that phosphorus as a single atom is unstable; we introduce phosphorus into our bodies in the form of phosphate. This is an important distinction because the terminology is often used interchangeably in the literature; in this review we have attempted to preserve this rhetorical precision.
EPIDEMIOLOGY AND CLINICAL OUTCOMES
Among hospitalized patients, hypophosphatemia (defined as serum phosphorus <2.5 mg/dL) is observed in up to 5% of individuals, but the rate is much higher in patients with advanced cancer. Based on a single-center report, the incidence of moderate hypophosphatemia (serum phosphorus <2 mg/dL) was 22.9% in ambulatory patients. In comparison, ˂1% of the general population is reported to have moderate hypophosphatemia [1, 2]. Of course, several factors, including the nutritional status and stage of cancer as well as therapy, influence the incidence of hypophosphatemia. The prevalence of hypophosphatemia in critically ill patients is >40%, and although it stands to reason that malignancy would raise the incidence, the epidemiology has not been well-characterized [3]. Nevertheless, in hospitalized patients, a study found that malignancy was the fourth leading etiology for severe hypophosphatemia (<1.5 mg/dL), accounting for 6% of cases [4]. Furthermore, multiple studies have demonstrated that severe hypophosphatemia (<1.5 mg/dL) is an adverse prognostic marker in hospitalized patients with increased healthcare utilization, in-hospital stays, postoperative complications, cost and mortality and lower quality of life [5–8].
The clinical presentation for hypophosphatemia varies depending on its duration and severity. In mild cases (2–2.4 mg/dL), patients may be asymptomatic. When patients have moderate or severe hypophosphatemia, symptoms may become more apparent. The effects of intracellular phosphate depletion are far-ranging and affect multiple organs, because it affects oxygen affinity to hemoglobin in red blood cells. Specifically, phosphate depletion (serum phosphorus <1 mg/dL) decreases 2,3-diphosphoglycerate levels, inhibiting oxygen release to tissues [9]. Depletion of phosphate also limits adenosine triphosphate (ATP) synthesis, which is vital for multiple intracellular functions and organ systems [10, 11]. Additional adverse hematological effects, which are rare, include inducing red blood cell rigidity and hemolysis [12]. Phosphate depletion (<1 mg/dL) can reduce the capacity for hemostasis, reducing platelet number and function. In leukocytes, it can impair chemotaxis and phagocytosis, affecting immunity [13]. Neurologically, very severe hypophosphatemia (<0.6 mg/dL) can contribute to paresthesia, altered mental status, encephalopathy, seizures and coma [14]. Depletion of ATP in the heart can result in depression of myocardial performance and tachyarrhythmias in patients with serum phosphate ˂1.5 mg/dL [15]. In the musculoskeletal system, chronic hypophosphatemia has been associated with increased bone resorption and osteomalacia (<1.5 mg/dL) and acute hypophosphatemia (<1.9 mg/dL) is associated with proximal myopathy, rhabdomyolysis, dysphagia and ileus [16]. In addition, hypophosphatemia with serum phosphorus <2.5 mg/dL and <1.5 mg/dL has been associated with respiratory muscle weakness in 70% and 100% of the general inpatient population, respectively, and in the critical care population, having a serum phosphorus level <2.5 mg/dL has been associated with an 18% greater risk of failure-to-wean from mechanical ventilation [17, 18].
PHYSIOLOGY OF PHOPHORUS HOMEOSTASIS
Phosphorus exists in our body in both organic and inorganic forms. Organic phosphorus is present with calcium (Ca2+) as hydroxyapatite in bones and teeth (85%) and intracellular in soft tissues (14%). Besides providing structure for the body, forming the extracellular matrix of bone and teeth, phosphorus is also a significant intracellular anion and an essential component of nucleic acids, cell membranes, receptor signaling proteins, enzymes, acid buffering and energy metabolism. Inorganic phosphates exist primarily as free phosphate ions in both divalent and monovalent H2 forms [19]. About 10% of inorganic phosphate is bound to plasma proteins and ∼5% is bound to cations such as sodium (Na+), Ca2+ and magnesium (Mg2+) [20]. The serum phosphorus level is homeostatically regulated and maintained in a narrow range between 3.0 and 4.5 mg/dL (0.97–1.45 mmol/L) by three principle hormones: parathyroid hormone (PTH), phosphatonins [mainly fibroblast growth factor 23 (FGF-23) and klotho] and 1,25-dihydroxyvitamin D3 [1,25-(OH)2D3] (calcitriol). Together, these hormones regulate how the intestine, kidneys and bones traffic phosphate.
Gastrointestinal (GI) absorption
The daily requirement of phosphorus in adults is 700 mg and the average Western diet consists of ∼20 mg/kg/day of phosphorus (Figure 1) [21]. Protein-rich diets, including meat, eggs, cereals and milk products, contain high amounts of phosphorus, and so does processed food [22]. Many medications contain phosphate as an excipient, which may not be reported on the package insert, but can provide a significant phosphorus burden [23, 24]. Of the total dietary intake, ∼60–75% is absorbed. Absorption occurs predominantly in the jejunum. Absorption is decreased in the presence of food rich in Ca2+, Mg2+ and aluminum. Phosphate absorption in the gut occurs through both paracellular and transcellular routes. Paracellular absorption is the predominant mechanism—the kinetics is linear, nonsaturable and dependent on the oral intake [25]. Transcellular absorption occurs through Na+–phosphate cotransporter type 2b (NaPi-2b), present in the intestinal microvilli [26]. Its expression is upregulated by low dietary phosphate [27] and calcitriol [28] and inhibited by niacin. PTH increases intestinal absorption of phosphate indirectly by increasing the production of calcitriol in the kidneys.
FIGURE 1:
Quantitative aspects of phosphorus homeostasis in humans [21]. Factors that can cause hypophosphatemia are in red.
KIDNEY HANDLING
Free and non-protein-bound phosphate is freely filtered at the level of the glomerulus and ∼85% is reabsorbed in the proximal tubule through secondary active transport by type 2a and c Na+–phosphate cotransporters (NaPi-2a and NaPi-2c) and Na+-dependent phosphate transporters 1 and 2 (PiT1 and PiT2) (Figure 2) [29]. NaPi-2a is located in the brush border membrane of the proximal convoluted tubule, which is most abundant on the S1 segment [30]. NaPi-2c is located throughout the proximal convoluted tubule. PiT1 and PiT2 make minor contributions to renal phosphate reabsorption.
FIGURE 2:
Mechanism for phosphate reabsorption in the proximal tubule in the kidney. Significant absorption occurs in the proximal tubule (85%) and ascending limb of the loop of Henle/distal convoluted tubule (15%). A−, organic anion.
About 5–20% of filtered phosphate (13 mg/kg/day) is eventually excreted in the urine (Figure 1). High serum phosphorus levels cause internalization of the transporters and an increase in phosphate excretion. Renal phosphate excretion is also increased by PTH and phosphatonins (including FGF-23 and klotho) through the downregulation of NaPi-2a. Phosphate excretion is also increased by glucocorticoids [31] and estrogen [32]. In the setting of hypophosphatemia, fractional excretion of phosphate >5% (or 24-h excretion >100 mg) is diagnostic of renal phosphate wasting [33]. Excretion is decreased by growth hormone, thyroid hormone, insulin [34] and insulin-like growth factor (IGF) [35]. The remainder of phosphate excretion occurs through feces, which contain the unabsorbed phosphate in the diet and the phosphate excreted in digestive juices and enzymes [21].
REGULATION BY PTH, VITAMIN D AND PHOSPHATONINS
PTH is an 84 amino acid polypeptide hormone secreted by the chief cells of the parathyroid gland. The primary stimulus for its secretion is a low serum Ca2+ level detected by Ca2+-sensing receptors. A high serum phosphorus level increases the PTH level, which acts on PTH receptor (PTHR) 1 in the proximal tubule, causing downregulation and removal of NaPi-2a, NaPi-2c and PiT-2 channels from the brush border membrane, resulting in renal phosphate loss [36, 37]. PTH upregulates 1-α-hydroxylase enzyme expression in the kidneys, resulting in an increased calcitriol level as described below. PTHR is also present in the bones, where it causes bone resorption and the release of Ca2+ and phosphate [38].
Vitamin D is essential for normal phosphate homeostasis and it is helpful to review the various forms and roles of its action. In the skin, ultraviolet B radiation present in sunlight converts 7-dehydrocholesterol to cholecalciferol, which gets hydroxylated to 25-hydroxy-cholecalciferol by 25-hydroxylase in the liver, and further hydroxylation occurs in the kidneys by 1-α hydroxylase enzyme to ultimately form calcitriol. This rate-limiting last step is induced by PTH, hypocalcemia and hypophosphatemia and inhibited by FGF-23, hyperphosphatemia and hypercalcemia [39, 40]. Calcitriol mainly acts on the kidneys to increase Ca2+ reabsorption. It may have some role in increasing intestinal phosphate absorption through NaPi-2b overexpression, especially during dietary phosphorus deficiency [28, 41].
FGF-23 is a 251 amino acid protein produced in osteocytes and osteoblasts in the bone. It was identified as the long-sought-after phosphaturic factor in tumor-induced osteomalacia and autosomal dominant hypophosphatemic rickets [18, 42, 43]. Its production is stimulated by increased dietary phosphorus intake [44, 45] and by calcitriol [46]. FGF-23, along with coreceptor klotho, acts on the proximal tubule to decrease phosphate reabsorption by downregulating NaPi-2a and NaPi-2c [42]. FGF-23 and PTH are the two main hormones that have a significant role in causing phosphate loss in urine.
ETIOLOGY OF HYPOPHOSPHATEMIA IN CANCER
Pseudohypophosphatemia
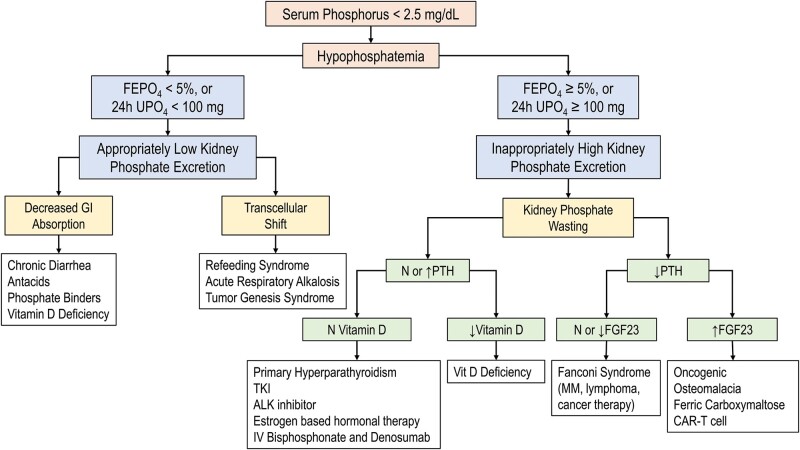
In general, the etiologies of hypophosphatemia in cancer can be classified by their mechanisms. However, it is important to be aware of pseudohypophosphatemia, defined as spuriously low serum phosphorus values that do not correspond to their actual systemic levels. Patients do not exhibit signs or symptoms of hypophosphatemia and no treatment is needed [47]. Pseudohypophosphatemia should be considered in patients receiving mannitol. It has an osmotic diuretic and a weak phosphaturic effect that can cause phosphate loss in urine, but more than that, it interferes with the colorimetric assay (DuPont aca endpoint method) for phosphorus by binding molybdate, decreasing both the rate of color development and the endpoint measurement [48]. Similarly, spurious hypophosphatemia should be in the differential of multiple myeloma (MM) and other paraproteinemias due to paraprotein’s interference with the phosphate assay [49]. Moreover, pseudohypophosphatemia was observed in acute leukemia patients with extreme hyperleukocytosis and was attributed to the increased metabolic activity of the leukemic cells in the testing tube. Phosphate levels normalized when transporting the test tube to the laboratory on ice [50]. Once spurious hypophosphatemia has been excluded, the diagnostic approach outlined in Figure 3 can be applied.
FIGURE 3:
Diagnostic approach to hypophosphatemia. FEPO4, fractional excretion of phosphate; 24-h UPO4, 24-h urine PO4; N, normal level; decreased.
Decreased intake
Patients with cancer frequently suffer from significant malnutrition marked by weight loss, loss of muscle mass and decreased activity. Treating malnutrition related to cancer and cancer therapy should be part and parcel of cancer treatment. Patients with cancer may have diminished appetite and hunger cues because of interrupted circadian rhythms or isolation. Patients with GI tumors or malignant ascites can experience early satiety, nausea and vomiting. Taste changes can frequently result from chemotherapy or radiation to the head and neck and create an aversion to food. Vulnerable patients with limited support systems may not have help with grocery shopping or preparing meals. Patients with cancer also suffer from depression, with negative symptoms resulting in anorexia.
Decreased intestinal absorption
In addition to inadequate intake, medications and chronic diarrhea contribute to poor intestinal absorption. For example, antacid and niacin can inhibit intestinal phosphate absorption. Aluminum- and Mg2+-based antacids can bind to both ingested and secreted phosphate in the intestine, while niacin promotes phosphate loss by reducing intestinal NaPi-2b expression [51, 52]. Hypophosphatemia associated with chronic diarrhea is typically mild or moderate in severity. It is a consequence of both poor intestinal absorption and urinary loss secondary to vitamin D deficiency with compensatory hyperparathyroidism. Examples of cancer therapy–associated diarrhea and hypophosphatemia include tyrosine kinase inhibitors (TKIs) and antitumor agents listed in Table 1.
Table 1.
Cancer therapies associated with hypophosphatemia
| Medication | Incidence (%) | Hypothesized mechanism |
|---|---|---|
|
1–16 [53–55] | Proximal tubule injury [55] |
|
Case reports [56–60] |
Proximal tubule injury Indirectly related to hypomagnesemia and hypocalcemia with associated vitamin D resistance and PTH secretion [56–60] |
|
66 [61] | Proximal tubule injury [61] |
|
13 [62] | Proximal tubule injury [62] |
|
Case reports [63–69] | Proximal tubule injury [63–69] |
|
Inhibition of PDGF receptor expressed in bone and proximal tubular cells [63,75,76] Vitamin D deficiency associated with chronic diarrhea [77] Unknown. Possibly related to inhibition of PDGF receptor expressed in bone and proximal tubular cells [63, 75, 76] |
|
|
3 [78] | Inhibition of IGF-1 receptor in proximal tubule [79] |
|
|
Unknown. Possibly related to activation of rapamycin-insensitive protein complex 2 (mTORC2) and klotho expression [81, 82] |
|
Described in case series [83] | Downregulation of type 2a Na+–phosphate cotransporter in proximal tubule secondary to estrogen effect [84] |
|
17 [85] | Unknown. A case report of Fanconi syndrome [86] |
|
37–57 [87] | Unknown. Possibly related to rising IL-6 that is associated with an increase in FGF-23 [87] |
Transcellular shift
Refeeding syndrome is a potentially fatal metabolic disturbance due to reinstitution of nutrition in patients who have experienced a period of poor nutrition. The period of malnutrition is typically at least 5 days but can be potentiated by metabolic stress or severe illness [88]. Shifts in potassium, Mg2+, acids and phosphorus can cause serious complications, including seizures, arrhythmia, heart failure and neurologic impairment. In malnutrition, hormonal and metabolic changes prevent protein breakdown of intracellular solutes, such as phosphate, and these become severely depleted. However, the serum phosphorus may remain normal because intracellular stores are being used and there is decreased renal excretion. During refeeding, insulin stimulates glycogen, fat and protein synthesis utilizing minerals, including phosphate, resulting in a precipitous decrease in serum phosphorus, potassium and Mg2+. The National Institute for Health and Care Excellence guidelines for nutritional support have been developed to ensure adequate identification and prevention of refeeding syndrome and prescribes calibrated refeeding with the proactive replacement of vitamins and electrolytes, including phosphate [89].
There are other examples of transcellular shifts, such as respiratory alkalosis, that can induce hypophosphatemia by increasing phosphofructokinase activity, consuming more phosphate for ATP production. Hypophosphatemia may also be a complication of hematologic malignancies in a phenomenon called tumor genesis syndrome [90], where hypophosphatemia is caused by a shift of extracellular phosphorus into the rapidly replicating malignant cells that consume extracellular stores [91, 92]. This phenomenon has been described in histiocytic lymphoma, Burkitt’s lymphoma and acute myelomonocytic leukemia. Similarly, patients after allogeneic stem cell transplants can develop hypophosphatemia during hematopoietic reconstitution [93].
Phosphaturia and Fanconi syndrome
Urinary phosphate wasting can be isolated as in patients with oncogenic osteomalacia, associated with bicarbonate loss as in patients with proximal renal tubular acidosis or in association with multiple solutes wasting as in Fanconi syndrome [94]. Certain hematological malignancies and cancer therapies have been associated with acquired proximal tubular dysfunction, leading to generalized impairment in reabsorption of solutes including phosphate, bicarbonate, glucose, potassium, uric acid and amino acids [95, 96].
Cancer-associated Fanconi syndrome
Fanconi syndrome occurs primarily in patients with lymphoma and monoclonal gammopathy. A few reports describe hypophosphatemia associated with Fanconi syndrome in patients with acute or relapsed adult T cell leukemia/lymphoma and Burkitt lymphoma. The etiology is attributed to infiltration of renal parenchyma by lymphomatous cells and a speculated role of human T lymphotropic virus 1, causing direct proximal tubule dysfunction [97, 98]. In patients with monoclonal gammopathy, the accumulation of light chains, mainly kappa light chains, in the proximal tubules can alter the cells’ proteolytic function, forming an intracellular crystal, or, through oxidative stress, can lead to cell dedifferentiation of the cells, apoptosis and subsequent loss of reabsorptive capacity [99, 100]. Nonetheless, Fanconi syndrome is considered a rare complication. In a single-center study, Fanconi syndrome was attributed to monoclonal gammopathy of undetermined significance in 44% of patients, MM in 31%, smoldering MM in 19% and Waldenstrom macroglobulinemia in 6% [101]. Hypophosphatemia can also be isolated with hyperphosphaturia only and without associated proximal tubule defects [102].
Cancer therapy–associated Fanconi syndrome
Ifosfamide enters proximal tubule cells through organic cation transporter 2 (OCT2) and is metabolized to chloroacetaldehyde, which induces glutathione depletion and subsequently oxidative damage [103]. The incidence of hypophosphatemia (<2.5 mg/dL) varies widely between studies, from 1 to 16% [53, 54]. Risk factors for nephrotoxicity and hypophosphatemia include a cumulative dose of ifosfamide >60 g/m2, young age and pretreatment with cisplatin. Furthermore, hypophosphatemia might persist or even develop months after cessation of ifosfamide therapy [55]. Medications associated with hypophosphatemia in cancer patients and their hypothesized mechanisms are summarized in Table 1.
Several chemotherapeutic drugs are notable for inducing Fanconi syndrome. Azacitidine is a hypomethylating agent used for the treatment of acute myelocytic leukemia. In a study evaluating the nephrotoxicity associated with azacitidine, hypophosphatemia (<2 mg/dL) developed in 66% of patients and was attributed to proximal tubular dysfunction. This dysfunction resolved rapidly after the completion of chemotherapy [61]. Streptozocin is an alkylating agent in the nitrosourea chemotherapy class used to treat multiple malignancies, including pancreatic neuroendocrine tumors. In two studies evaluating the toxicity of streptozocin in various hematological and solid organ malignancies, the incidence of hypophosphatemia (<2.4 mg/dL) was ~13%. The phosphorus level returned to normal within a few weeks of completing the therapy [62]. Cisplatin is an alkylating agent in the platinum chemotherapy class and is used to treat multiple solid organ malignancies. Numerous reports have described cisplatin’s association with various electrolyte disturbances, including hyponatremia, hypokalemia, hypomagnesemia, hypocalcemia and hypophosphatemia [104]. Solute wasting is in part related to cisplatin selective injury to S3 segment proximal tubular epithelial cells, where OCT2 plays a pivotal role in the uptake and accumulation of cisplatin in these cells [56, 57]. Another possible explanation for phosphate wasting is related to the concurrent hypomagnesemia and hypocalcemia that contribute to vitamin D resistance and stimulate PTH secretion [58, 59]. Other cancer therapies associated with hypophosphatemia attributed to proximal tubular injury/Fanconi-like syndrome based on case reports are listed in Table 1.
Oncogenic osteomalacia
Oncogenic osteomalacia, also known as tumor-induced osteomalacia (TIO), is typically the result of benign, slow-growing phosphaturic mesenchymal tumors (PMTs) and rarely manifest as a paraneoplastic feature of malignant disease [105]. These tumors secrete FGF-23 and cause hypophosphatemia through renal phosphate wasting [106]. Clinically patients present with bone pain, gait disturbances, pathological fractures, height loss and proximal muscle weakness [107]. Biochemically it is characterized by hypophosphatemia, normal or low calcitriol and elevated or inappropriately normal FGF-23. Chronic hypophosphatemia leads to osteomalacia that appears as osteopenia and pseudofractures on radiographs. The most common tumors causing TIO are the PMT mixed connective tissue variants [108]. They are usually small in size and may be located in any soft tissue or bone site throughout the body. Localization is difficult and may require an 18F-fluorodeoxyglucose positron emission tomography (PET) scan or octreotide scan [109]. More recent techniques of using somatostatin analog combined with a PET–computed tomography (CT) scan (gallium-68 dotatate PET-CT scan) have shown better results in detecting the tumor [110]. A venous sampling of the FGF-23 level may be needed if multiple possible sources are detected [111]. Surgery provides definitive treatment, but excision with wide margins is necessary to ensure complete removal since recurrence rates are high with incomplete resection [112]. Postoperatively the FGF-23 level decreases rapidly, with normalization of the phosphate level by postoperative Day 5, and skeletal changes may take up to a year to normalize [113].
Targeted and novel therapies causing hypophosphatemia
TKI
Imatinib is a BCR-ABL TKI used to treat Philadelphia chromosome–positive chronic myelogenous leukemia (CML). In two clinical trials, the incidence of hypophosphatemia (<2.5 mg/dL) associated with imatinib use in CML patients was ~50%. One-third of these patients had serum phosphorus <2 mg/dL [70]. Less commonly, hypophosphatemia with levels ˂2 mg/dL was reported in 13 and 1.9% of metastatic renal cell carcinoma patients treated with sorafenib and sunitinib, respectively [71, 72]. The observed hypophosphatemia was attributed to possible inhibition of platelet-derived growth factor receptor (PDGFR) expressed on proximal tubular cells, with subsequent tubular dysfunction and inhibition of PDGFR expressed on osteoclasts, resulting in decreased bone resorption and secondary hyperparathyroidism [63, 75]. Other multi-TKIs that target PDGFR include regorafenib, nilotinib and dasatinib. The reported incidence for hypophosphatemia (<2 mg/dL) with these TKIs was 5.2, 15 and 7%, respectively [64, 73, 74]. Additionally, ceritinib is a TKI that inhibits anaplastic lymphoma kinase (ALK) and IGF-1 receptor. Hypophosphatemia (<2 mg/dL) developed in 3% of patients treated with ceritinib and was attributed to possible phosphaturia. The mechanism for phosphaturia could be related to the inhibition of the insulin (IGF-1) receptor in proximal tubules that promotes phosphate reabsorption [78, 79].
Mammalian target of rapamycin (mTOR) inhibitors
Hypophosphatemia is a common finding in cancer patients treated with mTOR inhibitors. The reported incidence of <2 mg/dL was 13% with temsirolimus, 4% with everolimus and 21.7% with ridaforolimus [80]. Based on an observational study, the hypophosphatemia associated with everolimus and temsirolimus use, among other biological markers, was significantly associated with tumor response and progression-free survival in patients with renal cell carcinoma [114]. The mechanism for mTOR inhibitor–associated hypophosphatemia is still not identified. Nonetheless, sirolimus, an mTOR inhibitor, has been associated with impaired proximal tubular phosphate reabsorption in kidney allograft recipients. A postulated mechanism for this observed phosphate wasting is related to activation of rapamycin-insensitive protein complex 2 (mTORC2) and klotho expression by sirolimus [81].
Hormonal therapy
Estramustine and high-dose diethylstilbestrol diphosphate are estrogen-based medications used to treat metastatic prostate cancer and are associated with mild to moderate hypophosphatemia. Serum phosphorus levels typically decrease during the first 6 weeks of therapy then stabilize at a low normal range (2.6 ± 0.6 mg/dL). The level returns to normal upon discontinuation of treatment. The phosphaturia etiology is attributed to the estrogen effect on proximal tubules with downregulation of NaPi-2a [83, 84].
Immunotherapy
Hypophosphatemia is a commonly observed side effect after chimeric antigen receptor (CAR) T cell infusion. Fifty-one percent of patients reported developing hypophosphatemia (<2 mg/dL); however, no further evaluation was performed to identify the etiology. It is postulated that the increase in interleukin-6 (IL-6) associated with CAR T cell therapy might increase FGF-23 levels [87]. Less frequently, hypophosphatemia (<2 mg/dL) was reported in patients treated with immune checkpoint inhibitors (ICPIs), with an overall incidence of 17% [85]. The etiology is still under investigation and likely multifactorial, as only a few reports describe the association between ICPI and Fanconi syndrome [65, 86].
Supportive therapies associated with hypophosphatemia
Total parenteral nutrition (TPN)
TPN is frequently utilized in cancer patients with head and neck cancer, esophageal or GI cancers and those who have undergone major abdominal surgeries or prolonged ileus or bowel obstruction. Despite the inclusion of phosphate in the TPN, the development of hypophosphatemia (<2.5 mg/dL) is common, occurring in up to 40% of patients [115]. Hypophosphatemia typically occurs early after initiating therapy related to refeeding syndrome [116]. Vigilance, anticipation and early replacement of phosphate can prevent the development of severe hypophosphatemia [117].
Intravenous (IV) iron
In a recently published systematic review, hypophosphatemia (<2.5 mg/dL) was observed in up to 92% of patients with iron deficiency anemia treated with third-generation IV iron preparation, specifically ferric carboxymaltose (FCM). Hypophosphatemia was transient; however, case reports described chronic hypophosphatemia with osteomalacia in patients with chronic anemia requiring repeated IV iron infusion [118]. The proposed mechanism is FCM blocking the cleavage of intact FGF-23 (iFGF-23) to inactive cleaved FGF-23 and subsequently the increased iFGF-23 level leads to phosphaturia [119]. Additional supportive therapies causing hypophosphatemia are listed in Table 2.
Table 2.
Supportive therapies associated with hypophosphatemia
| Medication | Incidence (%) | Hypothesized mechanism |
|---|---|---|
| TPN | 11–60 [115] | Transcellular shift, refeeding syndrome and unmet requirements [115, 120] |
| IV iron/FCM | Up to 92 [118] | Interference with FGF-23 metabolism [119] |
|
2.1 in metastatic bone disease [121] | RANK ligand-receptor inhibition will lead to compensatory elevation of PTH that can contribute to hypophosphatemia by inhibiting proximal tubule reabsorption [122] |
|
Decreased proximal tubular reabsorption secondary to elevation of PTH during the abrupt decrease in serum Ca2+ [122] Decreased proximal tubular reabsorption secondary to elevation of PTH Proximal tubular injury [124] |
|
| CRRT | 27–78 [125] | Continuous dialytic removal of phosphate [125] |
Denosumab
Denosumab blocks the receptor activator of nuclear factor κB ligand to decrease osteoclastic bone resorption in patients with osteoporosis and metastatic bone disease. This blockage leads to compensatory elevation of PTH during the first month of injection, which can contribute to hypophosphatemia by inhibiting the proximal tubule reabsorption of phosphorus [122]. About 2.1% of cancer patients with metastatic bone disease treated with denosumab developed hypophosphatemia (<2.5 mg/dL) [121].
Intravenous IV bisphosphonates
The associated hypophosphatemia with IV bisphosphonates is usually mild, transient and attributed to the elevation of PTH during the abrupt decrease in serum Ca2+. The incidence varies based on the medication dose and the underlying disease. In patients with breast cancer and MM with metastatic bone disease treated with zoledronic acid and pamidronate, the reported hypophosphatemia (<2 mEq/L) incidence was 12.3 and 7.1%, respectively, while in patients with malignancy associated with hypercalcemia treated with zoledronic acid and desnosumab the reported incidence of hypophosphatemia was 2.1 and 1.1%, respectively [123].
Dialysis-related losses
Cancer patients receiving care in the intensive care unit (ICU) are at exceptionally high risk for hypophosphatemia. In addition to aspects of malignancy and its treatment contributing to diminished phosphorus stores, there are significant acid–base and electrolyte shifts that can contribute to hypophosphatemia. Furthermore, nutrition in the ICU is variable and patients may also be receiving dialytic therapy. Continuous renal replacement therapy (CRRT), in particular, is associated with hypophosphatemia, occurring in >50% of patients, and requires constant replacement [125]. Hypophosphatemia results from phosphate lost in the effluent during CRRT and can develop even if hyperphosphatemia is present at the start of therapy.
We summarized the common etiologies for hypophosphatemia in cancer patients based on the proposed mechanism in Figure 3.
MANAGEMENT
Hypophosphatemia in cancer patients should be corrected when it is first recognized and can be addressed by any member of the oncology team. In cases of severe or refractory hypophosphatemia, the involvement of nephrology should be considered. The general approach to hypophosphatemia should be to treat the underlying cause. In the case of antacids or diarrhea, for example, the agent can be discontinued and the diarrhea managed. In cases of a PMT, surgical resection usually results in resolution, but as noted, the tumors often recur. In patients with nonresectable FGF-23-secreting tumors, medical management consists of dietary phosphate and calcitriol supplementation. Hypocalcemia from phosphate therapy can give rise to secondary hyperparathyroidism, which necessitates allosteric inhibitors of calcium-sensing receptors like cinacalcet [126]. Recently an FGF-23 receptor monoclonal antibody, burosumab, has been approved for use in TIO [127, 128]. Acute conditions, such as respiratory alkalosis, should be corrected. Offending drugs like iron in bone mineralization therapy can be interrupted or replaced with alternatives while phosphorus is repleted. In critically ill patients receiving CRRT, phosphorus-containing dialysate, if available, should be considered and can be used to prevent the development proactively [129]. Decisions regarding agents specific for cancer are more difficult because, as much as possible, the general objective is to mitigate side effects while treating cancer, particularly if the offending agent is life-prolonging or curative. As a whole, hypophosphatemia, unless recurrent and life-threatening, should not be regarded as a reason to discontinue active cancer therapy, especially given the possibility of significantly improving the life expectancy of many cancer patients with the use of these agents.
Hypophosphatemia due to inadequate calorie intake, intolerance to diet or GI tract dysfunction may be addressed in part or whole with oral supplements or the implementation of enteral nutrition. Individualized recommendations containing phosphate-rich foods should be encouraged. If the dietary approach fails, then oral phosphate formulations with either Na+ phosphate or potassium phosphate salts should be used. Typical regimens required for oral supplementation range from 2.5 to 3.5 g (15 mg/kg) in three to four divided doses, and patients may require even higher doses when there is concomitant renal phosphate wasting [130]. Skim milk, if tolerated, is also an excellent source of phosphate replacement. Vitamin D is required for intestinal absorption; therefore, often nutritional and active vitamin D is required for optimal intestinal absorption. Frequently, antimotility and bulk-forming agents to manage diarrhea are needed while replacing phosphate.
In chronic cases where patients cannot introduce phosphate through the GI tract, parenteral replacement may be necessary. The risk of complications (i.e. hypocalcemia, hypotension, arrhythmia and acute kidney injury) is much higher than with oral phosphate due to the risk of precipitating with Ca2+ [131]. The response to parenteral phosphate is variable and not predicted well from initial levels, but general guidance has been published from several sources (Table 3). IV phosphate replacement should be considered in any symptomatic patient or severely depleted patient <1.0 mg/dL and patients should be monitored closely for the development of arrhythmias during the infusion and the development of hypocalcemia and hyperkalemia. Oral replacement can be resumed after symptoms resolve or serum phosphate is >1.5 mg/dL. Potential long-term consequences of phosphate replacement are extrapolated from other phosphate-wasting diseases, including nephrocalcinosis, chronic kidney disease and secondary hyperparathyroidism [132].
Table 3.
| Phosphorus level | Route | Weight 40–60 kg | Weight 61–80 kg | Weight 81–120 kg |
|---|---|---|---|---|
| <1.0 mg/dL | Initial management IV | 30 mmol phosphorus IV | 40 mmol phosphorus IV | 50 mmol phosphorus IV |
| 1.0–1.7 mg/dL | PO if asymptomatic and enteral route feasible; otherwise IV | 20 mmol phosphorus PO/IV | 30 mmol phosphorus PO/IV | 40 mmol phosphorus PO/IV |
| 1.8–2.2 mg/dL | PO if asymptomatic and enteral route feasible; otherwise IV | 10 mmol phosphorus PO/IV | 15 mmol phosphorus PO/IV | 20 mmol phosphorus PO/IV |
If the patient’s potassium is <4.0, use potassium phosphate; if the patient’s potassium is >4.0, use sodium phosphorus. Sodium phosphate injection provides the same phosphate content as potassium phosphate. The mmol conversion is 31 (e.g. 20 mmol = 620 mg); IV infusions should be given over 3–6 h or per hospital policy. PO, oral.
CONCLUSIONS
Hypophosphatemia is a common disorder in cancer patients that can result from malignancy itself or from its therapy. Poor intake, transcellular shift, GI and renal loss or RRT can all contribute to this condition. Hypophosphatemia can result in significant morbidity and mortality, including prolonged hospital stays and postoperative complications. An understanding of normal phosphate physiology is essential for prevention, identification of its etiology and treatment. Management can be complex, with treatment of underlying causes and oral and parenteral supplementation, and should be multidisciplinary.
CONFLICT OF INTEREST STATEMENT
None declared.
Contributor Information
Shreedhar Adhikari, Division of Renal-Electrolyte, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Omar Mamlouk, Section of Nephrology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Helbert Rondon-Berrios, Division of Renal-Electrolyte, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA.
Biruh T Workeneh, Section of Nephrology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
REFERENCES
- 1. Yoshida T, Taguchi D, Fukuda Ket al. Incidence of hypophosphatemia in advanced cancer patients: a recent report from a single institution. Int J Clin Oncol 2017; 22: 244–249 [DOI] [PubMed] [Google Scholar]
- 2. Betro MG, Pain RW.. Hypophosphataemia and hyperphosphataemia in a hospital population. Br Med J 1972; 1: 273–276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Halevy J, Bulvik S.. Severe hypophosphatemia in hospitalized patients. Arch Intern Med 1988; 148: 153–155 [PubMed] [Google Scholar]
- 4. Hoffmann M, Zemlin AE, Meyer WPet al. Hypophosphataemia at a large academic hospital in South Africa. J Clin Pathol 2008; 61: 1104–1107 [DOI] [PubMed] [Google Scholar]
- 5. Shor R, Halabe A, Rishver Set al. Severe hypophosphatemia in sepsis as a mortality predictor. Ann Clin Lab Sci 2006; 36: 67–72 [PubMed] [Google Scholar]
- 6. Zazzo JF, Troché G, Ruel Pet al. High incidence of hypophosphatemia in surgical intensive care patients: efficacy of phosphorus therapy on myocardial function. Intensive Care Med 1995; 21: 826–831 [DOI] [PubMed] [Google Scholar]
- 7. Giovannini I, Chiarla C, Nuzzo G.. Pathophysiologic and clinical correlates of hypophosphatemia and the relationship with sepsis and outcome in postoperative patients after hepatectomy. Shock 2002; 18: 111–115 [DOI] [PubMed] [Google Scholar]
- 8. Marik PE, Bedigian MK.. Refeeding hypophosphatemia in critically ill patients in an intensive care unit. A prospective study. Arch Surg 1996; 131: 1043–1047 [DOI] [PubMed] [Google Scholar]
- 9. Travis SF, Sugerman HJ, Ruberg RLet al. Alterations of red-cell glycolytic intermediates and oxygen transport as a consequence of hypophosphatemia in patients receiving intravenous hyperalimentation. N Engl J Med 1971; 285: 763–768 [DOI] [PubMed] [Google Scholar]
- 10. Larsen VH, Waldau T, Gravesen Het al. Erythrocyte 2,3-diphosphoglycerate depletion associated with hypophosphatemia detected by routine arterial blood gas analysis. Scand J Clin Lab Invest Suppl 1996; 224: 83–87 [DOI] [PubMed] [Google Scholar]
- 11. Subramanian R, Khardori R.. Severe hypophosphatemia. Pathophysiologic implications, clinical presentations, and treatment. Medicine (Baltimore) 2000; 79: 1–8 [DOI] [PubMed] [Google Scholar]
- 12. LaCelle PL. Alteration of membrane deformability in hemolytic anemias. Semin Hematol 1970; 7: 355–371 [PubMed] [Google Scholar]
- 13. Craddock PR, Yawata Y, VanSanten Let al. Acquired phagocyte dysfunction. A complication of the hypophosphatemia of parenteral hyperalimentation. N Engl J Med 1974; 290: 1403–1407 [DOI] [PubMed] [Google Scholar]
- 14. Silvis SE, Paragas PDJ.. Paresthesias, weakness, seizures, and hypophosphatemia in patients receiving hyperalimentation. Gastroenterology 1972; 62: 513–520 [PubMed] [Google Scholar]
- 15. O’Connor LR, Wheeler WS, Bethune JE.. Effect of hypophosphatemia on myocardial performance in man. N Engl J Med 1977; 297: 901–903 [DOI] [PubMed] [Google Scholar]
- 16. Singhal PC, Kumar A, Desroches Let al. Prevalence and predictors of rhabdomyolysis in patients with hypophosphatemia. Am J Med 1992; 92: 458–464 [DOI] [PubMed] [Google Scholar]
- 17. Gravelyn TR, Brophy N, Siegert Cet al. Hypophosphatemia-associated respiratory muscle weakness in a general inpatient population. Am J Med 1988; 84: 870–876 [DOI] [PubMed] [Google Scholar]
- 18. White KE, Evans WE, O’Riordan JLHet al. Autosomal dominant hypophosphataemic rickets is associated with mutations in FGF23. Nat Genet 2000; 26: 345–348 [DOI] [PubMed] [Google Scholar]
- 19. Iheagwara OS, Ing TS, Kjellstrand CMet al. Phosphorus, phosphorous, and phosphate. Hemodial Int 2013; 17: 479–482 [DOI] [PubMed] [Google Scholar]
- 20. Gaasbeek A, Meinders AE.. Hypophosphatemia: an update on its etiology and treatment. Am J Med 2005; 118: 1094–1101 [DOI] [PubMed] [Google Scholar]
- 21. Berndt TJ, Schiavi S, Kumar R.. “Phosphatonins” and the regulation of phosphorus homeostasis. Am J Physiol Renal Physiol 2005; 289: F1170–F1182 [DOI] [PubMed] [Google Scholar]
- 22. Carrigan A, Klinger A, Choquette SSet al. Contribution of food additives to sodium and phosphorus content of diets rich in processed foods. J Ren Nutr 2014; 24: 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li J, Wang L, Han Met al. The role of phosphate-containing medications and low dietary phosphorus-protein ratio in reducing intestinal phosphorus load in patients with chronic kidney disease. Nutr Diabetes 2019; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Nelson SML, Sarabia SRS, Christilaw Eet al. Phosphate-containing prescription medications contribute to the daily phosphate intake in a third of hemodialysis patients. J Ren Nutr 2017; 27: 91–96 [DOI] [PubMed] [Google Scholar]
- 25. Walton J, Gray TK.. Absorption of inorganic phosphate in the human small intestine. Clin Sci (Lond) 1979; 56: 407–412 [DOI] [PubMed] [Google Scholar]
- 26. Hilfiker H, Hattenhauer O, Traebert Met al. Characterization of a murine type II sodium-phosphate cotransporter expressed in mammalian small intestine. Proc Natl Acad Sci USA 1998; 95: 14564–14569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hattenhauer O, Traebert M, Murer Het al. Regulation of small intestinal Na-P(i) type IIb cotransporter by dietary phosphate intake. Am J Physiol 1999; 277: G756–G762 [DOI] [PubMed] [Google Scholar]
- 28. Danisi G, Bonjour JP, Straub RW.. Regulation of Na-dependent phosphate influx across the mucosal border of duodenum by 1,25-dihydroxycholecalciferol. Pflugers Arch 1980; 388: 227–232 [DOI] [PubMed] [Google Scholar]
- 29. Blaine J, Chonchol M, Levi M.. Renal control of calcium, phosphate, and magnesium homeostasis. Clin J Am Soc Nephrol 2015; 10: 1257–1272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magagnin S, Werner A, Markovich Det al. Expression cloning of human and rat renal cortex Na/Pi cotransport. Proc Natl Acad Sci USA 1993; 90: 5979–5983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Levi M, Shayman JA, Abe Aet al. Dexamethasone modulates rat renal brush border membrane phosphate transporter mRNA and protein abundance and glycosphingolipid composition. J Clin Invest 1995; 96: 207–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bansal N, Katz R, de Boer IHet al. Influence of estrogen therapy on calcium, phosphorus, and other regulatory hormones in postmenopausal women: the MESA study. J Clin Endocrinol Metab 2013; 98: 4890–4898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Walton RJ, Bijvoet OL.. Nomogram for derivation of renal threshold phosphate concentration. Lancet 1975; 2: 309–310 [DOI] [PubMed] [Google Scholar]
- 34. DeFronzo RA, Goldberg M, Agus ZS.. The effects of glucose and insulin on renal electrolyte transport. J Clin Invest 1976; 58: 83–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Biber J, Hernando N, Forster Iet al. Regulation of phosphate transport in proximal tubules. Pflugers Arch 2009; 458: 39–52 [DOI] [PubMed] [Google Scholar]
- 36. Bacic D, Lehir M, Biber Jet al. The renal Na+/phosphate cotransporter NaPi-IIa is internalized via the receptor-mediated endocytic route in response to parathyroid hormone. Kidney Int 2006; 69: 495–503 [DOI] [PubMed] [Google Scholar]
- 37. Segawa H, Yamanaka S, Onitsuka Aet al. Parathyroid hormone-dependent endocytosis of renal type IIc Na-Pi cotransporter. Am J Physiol Renal Physiol 2007; 292: F395–F403 [DOI] [PubMed] [Google Scholar]
- 38. Mannstadt M, Jüppner H, Gardella TJ.. Receptors for PTH and PTHrP: their biological importance and functional properties. Am J Physiol 1999; 277: F665–F675 [DOI] [PubMed] [Google Scholar]
- 39. Murayama A, Takeyama K, Kitanaka Set al. Positive and negative regulations of the renal 25-hydroxyvitamin D3 1α-hydroxylase gene by parathyroid hormone, calcitonin, and 1α,25(OH)2D3 in intact animals. Endocrinology 1999; 140: 2224–2231 [DOI] [PubMed] [Google Scholar]
- 40. Perwad F, Zhang MYH, Tenenhouse HSet al. Fibroblast growth factor 23 impairs phosphorus and vitamin D metabolism in vivo and suppresses 25-hydroxyvitamin D-1α-hydroxylase expression in vitro. Am J Physiol Renal Physiol 2007; 293: F1577–F1583 [DOI] [PubMed] [Google Scholar]
- 41. Segawa H, Kaneko I, Yamanaka Set al. Intestinal Na-P(i) cotransporter adaptation to dietary P(i) content in vitamin D receptor null mice. Am J Physiol Renal Physiol 2004; 287: F39–F47 [DOI] [PubMed] [Google Scholar]
- 42. Shimada T, Mizutani S, Muto Tet al. Cloning and characterization of FGF23 as a causative factor of tumor-induced osteomalacia. Proc Natl Acad Sci USA 2001; 98: 6500–6505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Jonsson KB, Zahradnik R, Larsson Tet al. Fibroblast growth factor 23 in oncogenic osteomalacia and X-linked hypophosphatemia. N Engl J Med 2003; 348: 1656–1663 [DOI] [PubMed] [Google Scholar]
- 44. Ferrari SL, Bonjour JP, Rizzoli R.. Fibroblast growth factor-23 relationship to dietary phosphate and renal phosphate handling in healthy young men. J Clin Endocrinol Metab 2005; 90: 1519–1524 [DOI] [PubMed] [Google Scholar]
- 45. Burnett SAM, Gunawardene SC, Bringhurst FRet al. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res 2006; 21: 1187–1196 [DOI] [PubMed] [Google Scholar]
- 46. Nishi H, Nii-Kono T, Nakanishi Set al. Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 2005; 101: c94–c99 [DOI] [PubMed] [Google Scholar]
- 47. Liamis G, Liberopoulos E, Barkas Fet al. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol 2013; 38: 50–57 [DOI] [PubMed] [Google Scholar]
- 48. Donhowe JM, Freier EF, Wong ETet al. Factitious hypophosphatemia related to mannitol therapy. Clin Chem 1981; 27: 1765–1769 [PubMed] [Google Scholar]
- 49. Wen Wu L, Choi TS, Barbosa Met al. Pseudohypophosphatemia in a patient with multiple myeloma. AACE Clin Case Rep 2020; 6: e334–e337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Polak R, Huisman A, Sikma MAet al. Spurious hypokalaemia and hypophosphataemia due to extreme hyperleukocytosis in a patient with a haematological malignancy. Ann Clin Biochem 2010; 47: 179–181 [DOI] [PubMed] [Google Scholar]
- 51. Lotz M, Zisman E, Bartter FC.. Evidence for a phosphorus-depletion syndrome in man. N Engl J Med 1968; 278: 409–415 [DOI] [PubMed] [Google Scholar]
- 52. Maccubbin D, Tipping D, Kuznetsova Oet al. Hypophosphatemic effect of niacin in patients without renal failure: a randomized trial. Clin J Am Soc Nephrol 2010; 5: 582–589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Oberlin O, Fawaz O, Rey Aet al. Long-term evaluation of Ifosfamide-related nephrotoxicity in children. J Clin Oncol 2009; 27: 5350–5355 [DOI] [PubMed] [Google Scholar]
- 54. Lee BS, Lee JH, Kang HGet al. Ifosfamide nephrotoxicity in pediatric cancer patients. Pediatr Nephrol 2001; 16: 796–799 [DOI] [PubMed] [Google Scholar]
- 55. Rossi R, Pleyer J, Schäfers Pet al. Development of ifosfamide-induced nephrotoxicity: prospective follow-up in 75 patients. Med Pediatr Oncol 1999; 32: 177–182 [DOI] [PubMed] [Google Scholar]
- 56. Filipski KK, Loos WJ, Verweij Jet al. Interaction of cisplatin with the human organic cation transporter 2. Clin Cancer Res 2008; 14: 3875–3880 [DOI] [PubMed] [Google Scholar]
- 57. Dobyan DC, Levi J, Jacobs Cet al. Mechanism of cis-platinum nephrotoxicity: II. morphologic observations. J Pharmacol Exp Ther 1980; 213: 551–556 [PubMed] [Google Scholar]
- 58. Ozsoylu S, Hanioğlu N.. Serum magnesium levels in children with vitamin D deficiency rickets. Turk J Pediatr 1977; 19: 89–96 [PubMed] [Google Scholar]
- 59. Paunier L. Effect of magnesium on phosphorus and calcium metabolism. Monatsschr Kinderheilkd 1992; 140(9 Suppl 1): S17–S20 [PubMed] [Google Scholar]
- 60. Anast CS, Forte LF.. Parathyroid function and magnesium depletion in the rat. Endocrinology 1983; 113: 184–189 [DOI] [PubMed] [Google Scholar]
- 61. Peterson BA, Collins AJ, Vogelzang NJet al. 5-Azacytidine and renal tubular dysfunction. Blood 1981; 57: 182–185 [PubMed] [Google Scholar]
- 62. Broder LE, Carter SK.. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med 1973; 79: 108–118 [DOI] [PubMed] [Google Scholar]
- 63. François H, Coppo P, Hayman JPet al. Partial fanconi syndrome induced by imatinib therapy: a novel cause of urinary phosphate loss. Am J Kidney Dis 2008; 51: 298–301 [DOI] [PubMed] [Google Scholar]
- 64. Yin X, Yin Y, Shen Cet al. Adverse events risk associated with regorafenib in the treatment of advanced solid tumors: meta-analysis of randomized controlled trials. Onco Targets Ther 2018; 11: 6405–6414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Farid S, Latif H, Nilubol Cet al. Immune checkpoint inhibitor-induced Fanconi syndrome. Cureus 2020; 12: e7686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Rago RP, Miles JM, Sufit RLet al. Suramin-induced weakness from hypophosphatemia and mitochondrial myopathy. Association of suramin with mitochondrial toxicity in humans. Cancer 1994; 73: 1954–1959 [DOI] [PubMed] [Google Scholar]
- 67. Buysschaert M, Cosyns JP, Barreto Let al. Pamidronate-induced tubulointerstitial nephritis with Fanconi syndrome in a patient with primary hyperparathyroidism. Nephrol Dial Transplant 2003; 18: 826–829 [DOI] [PubMed] [Google Scholar]
- 68. Denis D, Franck N, Fichel Fet al. Fanconi syndrome induced by vemurafenib: a new renal adverse event. JAMA Dermatol 2015; 151: 453–454 [DOI] [PubMed] [Google Scholar]
- 69. Shaikh A, Wiisanen ME, Gunderson HDet al. Acquired Fanconi syndrome after treatment with capecitabine, irinotecan, and bevacizumab. Ann Pharmacother 2009; 43: 1370–1373 [DOI] [PubMed] [Google Scholar]
- 70. Owen S, Hatfield A, Letvak L.. Imatinib and altered bone and mineral metabolism. N Engl J Med 2006; 355: 627; author reply 628–629 [DOI] [PubMed] [Google Scholar]
- 71. Escudier B, Eisen T, Stadler WMet al. Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 2007; 356: 125–134 [DOI] [PubMed] [Google Scholar]
- 72. Motzer RJ, Hutson TE, Tomczak Pet al. Overall survival and updated results for sunitinib compared with interferon alfa in patients with metastatic renal cell carcinoma. J Clin Oncol 2009; 27: 3584–3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Deremer DL, Ustun C, Natarajan K.. Nilotinib: a second-generation tyrosine kinase inhibitor for the treatment of chronic myelogenous leukemia. Clin Ther 2008; 30: 1956–1975 [DOI] [PubMed] [Google Scholar]
- 74. Jabbour E, Kantarjian HM, Saglio Get al. Early response with dasatinib or imatinib in chronic myeloid leukemia: 3-year follow-up from a randomized phase 3 trial (DASISION). Blood 2014; 123: 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Baldazzi V, Tassi R, Lapini Aet al. Sunitinib-induced hyperparathyroidism: a possible mechanism to altered bone homeostasis. Cancer 2012; 118: 3165–3172 [DOI] [PubMed] [Google Scholar]
- 76. Berman E, Nicolaides M, Maki RGet al. Altered bone and mineral metabolism in patients receiving imatinib mesylate. N Engl J Med 2006; 354: 2006–2013 [DOI] [PubMed] [Google Scholar]
- 77. Bellini E, Pia A, Brizzi MPet al. Sorafenib may induce hypophosphatemia through a fibroblast growth factor-23 (FGF23)-independent mechanism. Ann Oncol 2011; 22: 988–990 [DOI] [PubMed] [Google Scholar]
- 78. Kopple JD, Ding H, Hirschberg R.. Effects of recombinant human insulin-like growth factor 1 on renal handling of phosphorus, calcium, and sodium in normal humans. Am J Kidney Dis 1995; 26: 818–824 [DOI] [PubMed] [Google Scholar]
- 79. Shaw AT, Kim DW, Mehra Ret al. Ceritinib in ALK-rearranged non-small-cell lung cancer. N Engl J Med 2014; 370: 1189–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Soefje SA, Karnad A, Brenner AJ.. Common toxicities of mammalian target of rapamycin inhibitors. Target Oncol 2011; 6: 125–129 [DOI] [PubMed] [Google Scholar]
- 81. Tataranni T, Biondi G, Cariello Met al. Rapamycin-induced hypophosphatemia and insulin resistance are associated with mTORC2 activation and Klotho expression. Am J Transplant 2011; 11: 1656–1664 [DOI] [PubMed] [Google Scholar]
- 82. Schwarz C, Böhmig GA, Steininger Ret al. Impaired phosphate handling of renal allografts is aggravated under rapamycin-based immunosuppression. Nephrol Dial Transplant 2001; 16: 378–382 [DOI] [PubMed] [Google Scholar]
- 83. Citrin DL, Wallemark CB, Nadler Ret al. Estramustine affects bone mineral metabolism in metastatic prostate cancer. Cancer 1986; 58: 2208–2213 [DOI] [PubMed] [Google Scholar]
- 84. Faroqui S, Levi M, Soleimani Met al. Estrogen downregulates the proximal tubule type IIa sodium phosphate cotransporter causing phosphate wasting and hypophosphatemia. Kidney Int 2008; 73: 1141–1150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Seethapathy H, Rusibamayila N, Chute DFet al. Hyponatremia and other electrolyte abnormalities in patients receiving immune checkpoint inhibitors. Nephrol Dial Transplant 2020; doi: 10.1093/ndt/gfaa272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Tinawi M, Bastani B.. A case of Fanconi syndrome as a complication of treatment with a checkpoint inhibitor in a patient with hepatocellular carcinoma. J Nephropathol 2020; 9: e19 [Google Scholar]
- 87. Gupta S, Seethapathy H, Strohbehn IAet al. Acute kidney injury and electrolyte abnormalities after chimeric antigen receptor T-cell (CAR-T) therapy for diffuse large B-cell lymphoma. Am J Kidney Dis 2020; 76: 63–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Mehanna HM, Moledina J, Travis J.. Refeeding syndrome: what it is, and how to prevent and treat it. BMJ 2008; 336: 1495–1498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. National Collaborating Centre for Acute Care. Nutrition Support for Adults: Oral Nutrition Support, Enteral Tube Feeding and Parenteral Nutrition. London: National Collaborating Centre for Acute Care, 2006 [PubMed]
- 90. Wollner A, Shalit M, Brezis M.. Tumor genesis syndrome. Hypophosphatemia accompanying Burkitt’s lymphoma cell leukemia. Miner Electrolyte Metab 1986; 12: 173–175 [PubMed] [Google Scholar]
- 91. Zamkoff KW, Kirshner JJ.. Marked hypophosphatemia associated with acute myelomonocytic leukemia. Indirect evidence of phosphorus uptake by leukemic cells. Arch Intern Med 1980; 140: 1523–1524 [PubMed] [Google Scholar]
- 92. Matzner Y, Prococimer M, Polliack Aet al. Hypophosphatemia in a patient with lymphoma in leukemic phase. Arch Intern Med 1981; 141: 805–806 [PubMed] [Google Scholar]
- 93. Steiner M, Steiner B, Wilhelm Set al. Severe hypophosphatemia during hematopoietic reconstitution after allogeneic peripheral blood stem cell transplantation. Bone Marrow Transplant 2000; 25: 1015–1016 [DOI] [PubMed] [Google Scholar]
- 94. Liamis G, Milionis HJ, Elisaf M.. Medication-induced hypophosphatemia: a review. QJM 2010; 103: 449–459 [DOI] [PubMed] [Google Scholar]
- 95. Izzedine H, Launay-Vacher V, Isnard-Bagnis Cet al. Drug-induced Fanconi’s syndrome. Am J Kidney Dis 2003; 41: 292–309 [DOI] [PubMed] [Google Scholar]
- 96. Verzicco I, Regolisti G, Quaini Fet al. Electrolyte disorders induced by antineoplastic drugs. Front Oncol 2020; 10: 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Vanmassenhove J, Sallée M, Guilpain Pet al. Fanconi syndrome in lymphoma patients: report of the first case series. Nephrol Dial Transplant 2010; 25: 2516–2520 [DOI] [PubMed] [Google Scholar]
- 98. Goldsweig HG, Brisson de Champlain ML, Davidman M.. Proximal tubulare dysfunction associated with Burkitt’s lymphoma. Cancer 1978; 41: 568–577 [DOI] [PubMed] [Google Scholar]
- 99. Luciani A, Sirac C, Terryn Set al. Impaired lysosomal function underlies monoclonal light chain-associated renal Fanconi syndrome. J Am Soc Nephrol 2016; 27: 2049–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Sanders PW. Mechanisms of light chain injury along the tubular nephron. J Am Soc Nephrol 2012; 23: 1777–1781 [DOI] [PubMed] [Google Scholar]
- 101. Ma CX, Lacy MQ, Rompala JFet al. Acquired Fanconi syndrome is an indolent disorder in the absence of overt multiple myeloma. Blood 2004; 104: 40–42 [DOI] [PubMed] [Google Scholar]
- 102. Dash T, Parker MG, Lafayette RA.. Profound hypophosphatemia and isolated hyperphosphaturia in two cases of multiple myeloma. Am J Kidney Dis 1997; 29: 445–448 [DOI] [PubMed] [Google Scholar]
- 103. Springate J, Chan K, Lu Het al. Toxicity of ifosfamide and its metabolite chloroacetaldehyde in cultured renal tubule cells. In Vitro Cell Dev Biol Anim 1999; 35: 314–317 [DOI] [PubMed] [Google Scholar]
- 104. Yao X, Panichpisal K, Kurtzman Net al. Cisplatin nephrotoxicity: a review. Am J Med Sci 2007; 334: 115–124 [DOI] [PubMed] [Google Scholar]
- 105. Ramon I, Kleynen P, Valsamis Jet al. Hypophosphatemia related to paraneoplastic Cushing syndrome in prostate cancer: cure after bilateral adrenalectomy. Calcif Tissue Int 2011; 89: 442–445 [DOI] [PubMed] [Google Scholar]
- 106. Chong WH, Molinolo AA, Chen CCet al. Tumor-induced osteomalacia. Endocr Relat Cancer 2011; 18: R53–R77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Feng J, Jiang Y, Wang Oet al. The diagnostic dilemma of tumor induced osteomalacia: a retrospective analysis of 144 cases. Endocr J 2017; 64: 675–683 [DOI] [PubMed] [Google Scholar]
- 108. Folpe AL, Fanburg-Smith JC, Billings SDet al. Most osteomalacia-associated mesenchymal tumors are a single histopathologic entity: an analysis of 32 cases and a comprehensive review of the literature. Am J Surg Pathol 2004; 28: 1–30 [DOI] [PubMed] [Google Scholar]
- 109. Dupond JL, Mahammedi H, Prié Det al. Oncogenic osteomalacia: diagnostic importance of fibroblast growth factor 23 and F-18 fluorodeoxyglucose PET/CT scan for the diagnosis and follow-up in one case. Bone 2005; 36: 375–378 [DOI] [PubMed] [Google Scholar]
- 110. Clifton-Bligh RJ, Hofman MS, Duncan Eet al. Improving diagnosis of tumor-induced osteomalacia with Gallium-68 DOTATATE PET/CT. J Clin Endocrinol Metab 2013; 98: 687–694 [DOI] [PubMed] [Google Scholar]
- 111. Andreopoulou P, Dumitrescu CE, Kelly MHet al. Selective venous catheterization for the localization of phosphaturic mesenchymal tumors. J Bone Miner Res 2011; 26: 1295–1302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Sun Z, Qiu JJ, Gao Get al. Surgical treatment of tumor-induced osteomalacia: a retrospective review of 40 cases with extremity tumors. BMC Musculoskelet Disord 2015; 16: 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Chong WH, Andreopoulou P, Chen CCet al. Tumor localization and biochemical response to cure in tumor-induced osteomalacia. J Bone Miner Res 2013; 28: 1386–1398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Jebali M, Elaidi R, Brizard Met al. Biological toxicities as surrogate markers of efficacy in patients treated with mTOR inhibitors for metastatic renal cell carcinoma. BMC Cancer 2017; 17: 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Thompson JS, Hodges RE.. Preventing hypophosphatemia during total parenteral nutrition. JPEN J Parenter Enteral Nutr 1984; 8: 137–139 [DOI] [PubMed] [Google Scholar]
- 116. Marvin VA, Brown D, Portlock Jet al. Factors contributing to the development of hypophosphataemia when refeeding using parenteral nutrition. Pharm World Sci 2008; 30: 329–335 [DOI] [PubMed] [Google Scholar]
- 117. Fernandes VPI, Pinto Eal da C, Boin I de FSFet al. Phosphorus levels during infusion of parenteral nutrition with calorie-based phosphorus concentration: a case series. J Clin Nutr Metab 2009; 4: e252–e256 [Google Scholar]
- 118. Glaspy JA, Lim-Watson MZ, Libre MAet al. Hypophosphatemia associated with intravenous iron therapies for iron deficiency anemia: a systematic literature review. Ther Clin Risk Manag 2020; 16: 245–259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Kassianides X, Bhandari S.. Hypophosphataemia, fibroblast growth factor 23 and third-generation intravenous iron compounds: a narrative review. Drugs Context 2021; 10: 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Mashima Y, Ogawa M, Aoki Yet al. Changes in phosphorus distribution during total parenteral nutrition. J Parenter Enteral Nutr 1981; 5: 189–192 [DOI] [PubMed] [Google Scholar]
- 121. Lipton A, Fizazi K, Stopeck ATet al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer 2012; 48: 3082–3092 [DOI] [PubMed] [Google Scholar]
- 122. Bergwitz C, Jüppner H.. Regulation of phosphate homeostasis by PTH, vitamin D, and FGF23. Annu Rev Med 2010; 61: 91–104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Tanvetyanon T, Stiff PJ.. Management of the adverse effects associated with intravenous bisphosphonates. Ann Oncol 2006; 17: 897–907 [DOI] [PubMed] [Google Scholar]
- 124. Lu KC, Yeung LK, Lin SHet al. Acute effect of pamidronate on PTH secretion in postmenopausal hemodialysis patients with secondary hyperparathyroidism. Am J Kidney Dis 2003; 42: 1221–1227 [DOI] [PubMed] [Google Scholar]
- 125. Bellomo R, Cass A, Cole Let al. Intensity of continuous renal-replacement therapy in critically ill patients. N Engl J Med 2009; 361: 1627–1638 [DOI] [PubMed] [Google Scholar]
- 126. Geller JL, Khosravi A, Kelly MHet al. Cinacalcet in the management of tumor-induced osteomalacia. J Bone Miner Res 2007; 22: 931–937 [DOI] [PubMed] [Google Scholar]
- 127. Day AL, Gutiérrez OM, Guthrie BLet al. Burosumab in tumor-induced osteomalacia: a case report. Joint Bone Spine 2020; 87: 81–83 [DOI] [PubMed] [Google Scholar]
- 128. Jan de Beur SM, Miller PD, Weber TJet al. Burosumab for the treatment of tumor-induced osteomalacia. J Bone Miner Res 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Besnard N, Serveaux M, Machado Set al. Electrolytes-enriched hemodiafiltration solutions for continuous renal replacement therapy in acute kidney injury: a crossover study. Blood Purif 2016; 42: 18–26 [DOI] [PubMed] [Google Scholar]
- 130. Oronsky B, Caroen S, Oronsky Aet al. Electrolyte disorders with platinum-based chemotherapy: mechanisms, manifestations and management. Cancer Chemother Pharmacol 2017; 80: 895–907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131. Brunelli SM, Goldfarb S.. Hypophosphatemia: clinical consequences and management. J Am Soc Nephrol 2007; 18: 1999–2003 [DOI] [PubMed] [Google Scholar]
- 132. Taylor A, Sherman NH, Norman ME.. Nephrocalcinosis in X-linked hypophosphatemia: effect of treatment versus disease. Pediatr Nephrol 1995; 9: 173–175 [DOI] [PubMed] [Google Scholar]
- 133. Taylor BE, Huey WY, Buchman TGet al. Treatment of hypophosphatemia using a protocol based on patient weight and serum phosphorus level in a surgical intensive care unit. J Am Coll Surg 2004; 198: 198–204 [DOI] [PubMed] [Google Scholar]