Abstract
Background
Hyperkalaemia (HK) is a common electrolyte disorder in patients with chronic kidney disease (CKD) and/or treated with renin–angiotensin–aldosterone system inhibitors (RAASis). The aim of this study is to determine the severity, current management and cost of chronic HK.
Methods
We performed a retrospective cohort study of patients with chronic HK and CKD, heart failure or diabetes mellitus between 2011 and 2018. The study follow-up was 36 months.
Results
A total of 1499 patients with chronic HK were analysed: 66.2% presented with mild HK, 23.4% with moderate HK and 10.4% with severe HK. The severity was associated with CKD stage. Most patients (70.4%) were on RAASi therapies, which were frequently discontinued (discontinuation rate was 39.8, 49.8 and 51.8% in mild, moderate and severe HK, respectively). This RAASi discontinuation was similar with or without resin prescription. Overall, ion-exchange resins were prescribed to 42.5% of patients with HK and prescriptions were related to the severity of HK, being 90% for severe HK. Adherence to resin treatment was very low (36.8% in the first year and 17.5% in the third year) and potassium remained elevated in most patients with severe HK. The annual healthcare cost per patient with HK was €5929, reaching €12 705 in severe HK. Costs related to HK represent 31.9% of the annual cost per HK patient and 58.8% of the specialized care cost.
Conclusions
HK was usually managed by RAASi discontinuation and ion-exchange resin treatment. Most patients with HK were non-adherent to resins and those with severe HK remained with high potassium levels, despite bearing elevated healthcare expenditures.
Keywords: chronic kidney disease, diabetes, heart failure, hyperkalaemia, renin–angiotensin–aldosterone system inhibitors
Graphical Abstract
Graphical Abstract.
INTRODUCTION
Hyperkalaemia (HK) is a common electrolyte disorder characterized by increased serum potassium concentration >5.0 mEq/L [1] and increased risk for life-threatening events such as cardiac arrhythmias and sudden death [2]. In fact, HK is associated with an increased risk of all-cause mortality [3]. The prevalence of HK varies between 2.7% and 39%, being more prevalent in patients with comorbidities such as chronic kidney disease (CKD), diabetes mellitus or heart failure (HF) [4–8]. A study conducted in Spain in patients with chronic cardiovascular, metabolic and renal conditions reported a prevalence of HK of 2.7% [4]. The NEFRONA study in CKD patients reported an HK prevalence of 5.1 to 11.6% [9]; similarly, Crespo-Leiro et al. [8] observed rates in HF patients between 4.3% and 8.2%.
HK is usually associated with a loss of renal function, since as much as 90% of potassium is eliminated by urine. CKD, diabetes mellitus and the use of renin–angiotensin–aldosterone system inhibitors (RAASis) [10, 11] are major risk factors for HK. Nevertheless, main guidelines for the treatment of CKD [Kidney Disease: Improving Global Outcomes (KDIGO) and Kidney Disease Outcomes Quality Initiative] and HF (European Society of Cardiology, American College of Cardiology Foundation, American Heart Association and Heart Failure Society of America) recommend the use of RAASis [12] to reduce disease progression and increase survival in patients with advanced CKD (Stage ≥3), diabetes or chronic HF [1, 13–22].
Treatment strategies for HK are classified as acute (emergency) or chronic (maintenance). Treatment of acute or life-threatening HK is well-defined, including the haemodialysis indications [14]. However, the chronic management of HK is challenging.
The main objective of acute/emergency treatment of HK is to avoid potentially fatal arrhythmias. This is achieved by stabilizing the myocardial cell membranes, promoting the movement of extracellular potassium into the cell and encouraging the elimination of this cation from the body in the shortest possible time. In contrast, the objective of chronic treatment is to maintain stable serum potassium levels in the long term.
However, current treatments for HK have numerous restrictions and limitations that make their use in clinical practice difficult and, in turn, increase uncertainty about efficacy and safety. Intermittent treatment with cation exchange resins [calcium polystyrene sulfonate (CPS) and sodium polystyrene sulfonate (SPS)] is used, although it has important limitations [7], such as poor tolerability, severe gastrointestinal complications including intestinal necrosis, risk of hypokalaemia and lack of long-term efficacy and safety studies [7, 8]. Moreover, a recent study reported that approximately one-quarter of the patients receiving CPS do not adhere to treatment [23]. Consequently, in the absence of effective pharmacological alternatives for the management of HK, many doctors prefer to avoid this treatment option and decide to reduce or stop RAASi [24].
Accordingly, a study from Spain showed that only 25.9% of patients received target doses of RAASi, mainly because of HK, thus precluding the benefit of RAASi treatment demonstrated in clinical trials both in diabetic and non-diabetic CKD and in HF patients with reduced ejection fraction [24]. Ouwerkerk et al. [25] reported that reaching <50% of the recommended angiotensin-converting enzyme inhibitor/angiotensin receptor blocker and β-blocker dose was associated with an increased risk of death and/or HF hospitalization. In line with these results, Epstein et al. [26] reported that cardiorenal adverse events/mortality and mortality occurred in 34.3 and 11.0% of patients who discontinued RAASis and 24.9 and 8.2% of patients on submaximum doses [26].
HK was associated with increasing costs in the US Medicare system [27]. However, there is a lack of studies describing HK management, treatment adherence and economic burden on the National Health System from any European country.
Thus the main objective of this study was to describe the usual management of chronic HK in patients with CKD, HF or diabetes mellitus, the impact of HK on the use of RAASis, the level of adherence to CPS or SPS and the economic impact of chronic HK on the Spanish health system.
MATERIALS AND METHODS
Design
This was a retrospective cohort study. The data were extracted from the dissociated and anonymized database BIG-PAC and managed by Big Data Healthcare specialists on Real Life Data [28]. The BIG-PAC database contains data of 1.9 million patients treated in health centres in Spain, which includes seven regional health systems. This database is part of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, a network of institutions coordinated by the European Medicines Agency. The members of this network are public institutions and contract and research organizations involved in research in pharmacoepidemiology and pharmacovigilance. According to the Organic Law on Data Protection (Organic Law 3/2018 of 5 December), the BIG-PAC database lacked information that would allow identification of the patient. The study was classified by the Spanish Agency for Medicines and Healthcare Products as EPA-OD (Post Authorization Studies Other Designs) and was subsequently approved by the Clinical Research Ethics Committee of Bellvitge University Hospital on 24 October 2019 (reference PR339/19).
Study population
Adult patients (≥18 years) diagnosed with chronic HK (defined as at least two episodes of HK in 12 months, separated by at least 2 weeks) and diagnosed with CKD, heart failure or diabetes mellitus that required healthcare for an episode of HK between 1 January 2011 and 31 December 2018 in seven of the Spanish regional health systems were included. The study follow-up period was 36 months. Exclusion criteria were the following: CKD Stage 5, potassium supplements, Addison’s disease, congenital adrenal hyperplasia, hyperkalaemic periodic paralysis, patients with missing or inconsistent data and patient death during the study period (Figure 1).
FIGURE 1:
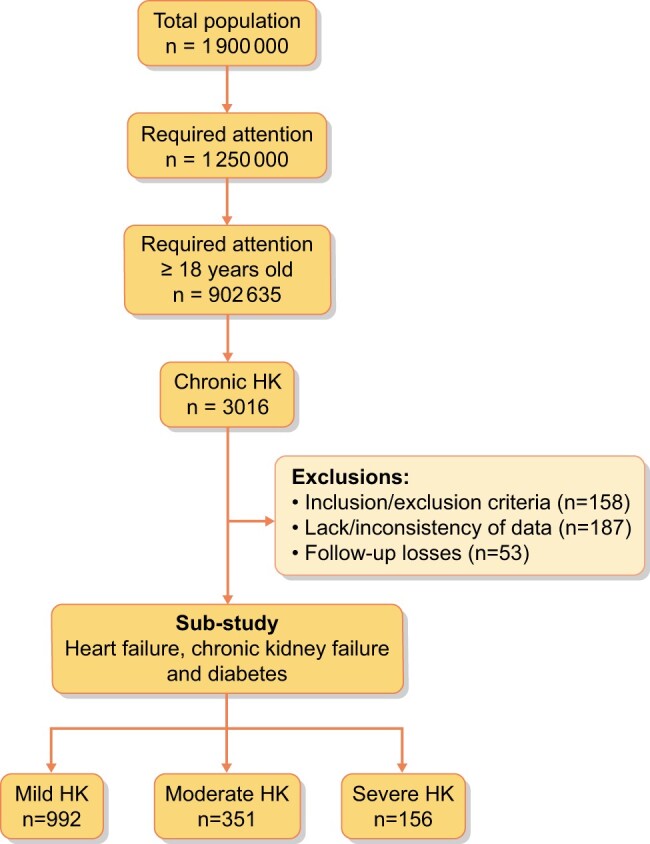
Patient flow chart.
Study variables
Chronic HK was considered as a dependent variable and was categorized as mild (5.1–5.4 mEq/L), moderate (5.5–6.0 mEq/L) or severe (≥6.1 mEq/L) [1]. Sociodemographic variables included age and sex. Clinical variables included systolic and diastolic blood pressure (mmHg), body mass index, heart failure severity, Charlson comorbidity index (CCI), serum creatinine, glomerular filtration rate, renal disease stage and associated comorbidities. Variables associated with the use of health services due to HK included hospitalization, emergency department medical visits and medical specialist visits whose codes were related to HK.
Measuring instruments
We used the following measuring instruments: CCI (a description of a patient's life expectancy at 10 years based on the presence of comorbidities and other factors such as age) [29, 30], the New York Heart Association scale of heart failure severity rating [31], treatment adherence [32] (percentage of prescriptions collected from the pharmacy office in relation to the total number of prescriptions) with CPS or SPS in the different HK severity groups and costs, which were obtained from regional tariffs lists [33] and from the National Institute of Statistics (INE) [34].
Data analysis and statistics
A descriptive statistical analysis was performed using absolute and relative frequencies for the qualitative data, while the quantitative data were described using the mean and standard deviation (SD). The normality of the distributions was checked with the Kolmogorov–Smirnov test and the adherence was quantified by applying Kaplan–Meier survival curves (median time). For comparisons between actuarial curves, the tablecloth–Cox log-rank test was used. In the bivariate analysis, the Student’s t-test, analysis of variance, chi-square and non-parametric Mann–Whitney–Wilcoxon test were used.
To determine the costs of the patients with HK, we obtained data about the use of resources from the database and we used unit costs from regional tariffs lists and from the INE. Prescriptions (acute, chronic or upon request) were quantified according to the recommended retail price per package at the time of prescription (Bot Plus database) [35]. The total cost was expressed as the mean cost per patient (annual mean). The mean use of resources per patient was obtained from the database and was multiplied by the unit costs for each resource to obtain the total costs per patient. These costs have been calculated for all the subgroups taken into account during the study (mild, moderate , and severe HK and total population included in the study).
Finally, a multivariable analysis evaluated the incremental cost burden associated with HK severity. Specifically, generalized linear models with gamma error distribution and log link were fit to the data. Explanatory variables included HK severity, age, sex, concomitant medication, resins treatment and prevalent comorbid conditions.
RESULTS
Basal and clinical characteristics
A total of 1499 patients diagnosed with chronic HK were included, of which 992 (66.2%) had mild HK, 351 (23.4%) had moderate HK and 156 (10.4%) had severe HK (Table 1). Of the total population, 54.4% were women and the mean age was 75.8 ± 9.2 years. The mean CCI was 3.1, with an average of 4.0 comorbidities per patient. The most frequent comorbidities were hypertension, diabetes mellitus, dyslipaemia, CKD, HF and obesity. As expected, the severity of HK was directly related to the severity of CKD and HF. As shown in Table 2, 70.4% of patients with HK were taken RAASis.
Table 1.
Baseline characteristics (demographics and morbidity) of the sample by study groups
| Characteristics | Total | Mild HK | Moderate HK | Severe HK | P-value |
|---|---|---|---|---|---|
| (N = 1499) | (n = 992) | (n = 351) | (n = 156) | ||
| Sociodemographics | |||||
| Age (years), mean (SD) | 75.8 (9.2) | 75.6 (8.7) | 76.1 (10.5) | 76.8 (9.5) | <0.001 |
| Range (years), % | |||||
| 18–44 | 0.7 | 0.4 | 1.4 | 0.6 | 0.175 |
| 45–64 | 11.3 | 11.8 | 9.7 | 12.2 | |
| 65–74 | 23.9 | 23.9 | 24.5 | 22.4 | |
| ≥75 | 64.1 | 63.9 | 64.4 | 64.7 | |
| Sex (female), % | 54.4 | 53.6 | 56.7 | 53.8 | 0.738 |
| General comorbidity | |||||
| Number of diagnoses, mean (SD) | 4.0 (1.6) | 4.0 (1.5) | 4.0 (1.6) | 4.3 (1.7) | <0.001 |
| CCI, n (%( | 3.1 (1.7) | 3.0 (1.7) | 3.1 (1.8) | 3.5 (1.8) | <0.001 |
| 1 | 21.9 | 23.5 | 20.5 | 14.7 | 0.005 |
| 2 | 20.3 | 20.4 | 21.7 | 17.3 | |
| 3+ | 57.8 | 56.1 | 57.8 | 67.9 | |
| Associated comorbidities, % | |||||
| Hypertension | 76.7 | 76.7 | 77.2 | 75.0 | 0.085 |
| Diabetes mellitus | 73.3 | 74.8 | 69.5 | 72.4 | 0.233 |
| Dyslipaemia | 54.4 | 55.4 | 51.9 | 53.2 | 0.885 |
| Kidney failure (GF <60) | 44.4 | 41.2 | 49.0 | 54.5 | <0.001 |
| Heart failure | 32.4 | 32.2 | 31.1 | 36.5 | 0.086 |
| Obesity | 28.8 | 30.5 | 24.5 | 26.9 | 0.981 |
| Ischaemic heart disease | 24.9 | 25.0 | 25.1 | 24.4 | 0.474 |
| Asthma and COPD | 23.5 | 22.8 | 23.1 | 28.8 | 0.018 |
| Stroke | 13.0 | 13.1 | 11.7 | 15.4 | 0.242 |
| Depressive syndrome | 12.0 | 9.9 | 14.5 | 19.9 | <0.001 |
| Malignant neoplasms | 9.8 | 8.9 | 10.5 | 14.1 | 0.194 |
| Active smokers | 8.5 | 9.0 | 7.4 | 7.7 | 0.138 |
| Anthropometric–biochemical parameters, mean (SD) | |||||
| Systolic blood pressure (mmHg) | 133.4 (18.9) | 134.0 (19.0) | 132.0 (19.4) | 132.8 (16.8) | 0.279 |
| Diastolic blood pressure (mmHg) | 79.0 (11.8) | 78.9 (11.8) | 78.7 (12.0) | 79.8 (11.1) | 0.958 |
| Body mass index (kg/m2) | 29.1 (4.6) | 29.3 (4.8) | 28.6 (4.1) | 29.3 (4.4) | 0.059 |
| Glomerular filtration rate (mL/min/1.73 m2) | 67.9 (24.0) | 69.6 (22.5) | 67.0 (25.5) | 59.0 (27.2) | <0.001 |
| Kidney disease stage, % | |||||
| CKD 1 | 20.5 | 21.1 | 23.6 | 9.6 | 0.078 |
| CKD 2 | 35.5 | 38.3 | 27.4 | 35.9 | 0.097 |
| CKD 3 | 37.2 | 34.2 | 41.9 | 45.5 | <0.001 |
| CKD 4 | 6.9 | 6.5 | 7.1 | 9.0 | 0.149 |
| NYHA scale, % | |||||
| 1 | 33.8 | 36.1 | 34.9 | 19.3 | 0.173 |
| 2 | 27.2 | 27.3 | 23.9 | 33.3 | 0.043 |
| 3 | 27.2 | 21.9 | 36.0 | 40.4 | 0.235 |
| 4 | 11.8 | 14.8 | 5.2 | 7.0 | 0.038 |
P: statistical significance (difference between mild HK and severe HK; CKD1: CKD-EPI 91–≤130 mL/min/1.73 m²; CKD 2: CKD-EPI 59–≤90 mL/min/1.73 m²; CKD 3: CKD-EPI 31–≤60 mL/min/1.73 m²; CKD4: CKD-EPI 15–30 mL/min/1.73 m²; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration (refers to the equation used to assess estimated glomerular filtration rate); COPD, chronic obstructive pulmonary disease; GF, glomerular filtration; NYHA, New York Heart Association.
Table 2.
Use of resins (CPS and SPS) and concomitant medication related to HK
| Total (N = 1499) | Mild HK (n = 992) | Moderate HK (n = 351) | Severe HK (n = 156) | P-value | |
|---|---|---|---|---|---|
| Prescription of CPS or SPS | |||||
| Patients, % | 42.5 | 30.3 | 55.7 | 89.9 | <0.001 |
| Concomitant medication related to HK | |||||
| Number of drugs, mean (SD) | 1.6 (1.0) | 1.6 (1.0) | 1.6 (1.0) | 1.6 (1.1) | 0.248 |
| C09—Renin–angiotensin system, % | 70.4 | 71.4 | 70.4 | 64.7 | 0.712 |
| C03C—Loop diuretics, % | 36.6 | 34.8 | 37.9 | 44.9 | <0.001 |
| C07A—β-blockers, % | 31.9 | 31.9 | 30.5 | 35.3 | 0.195 |
| M01A—Anti-inflammatory and anti-rheumatic, % | 19.3 | 20.6 | 17.9 | 14.1 | 0.078 |
| C03E—Diuretics and potassium-sparing products,a % | 0.8 | 0.8 | 0.9 | 0.6 | 0.703 |
P: statistical significance (difference between mild HK and severe HK).
ATC code C03E group includes mineralocorticoid receptor antagonist.
Use of resins and adherence to treatment
Ion-exchange resins were frequently prescribed in patients with HK (Table 2), directly related with its severity. Indeed, resins were prescribed in nearly 90% of patients with severe HK.
The mean duration of resin treatment per year depended on the severity of HK and was 85, 140 and 181 days in patients with mild, moderate and severe HK, respectively. However, only one-third of the patients (36.8%) were adherent to resins during the first year. This percentage decreased over time and was very low at the end of the study, with only 17.5% of patients adherent in the third year (Figure 2). Furthermore, resin adherence was associated with the severity of HK.
FIGURE 2:
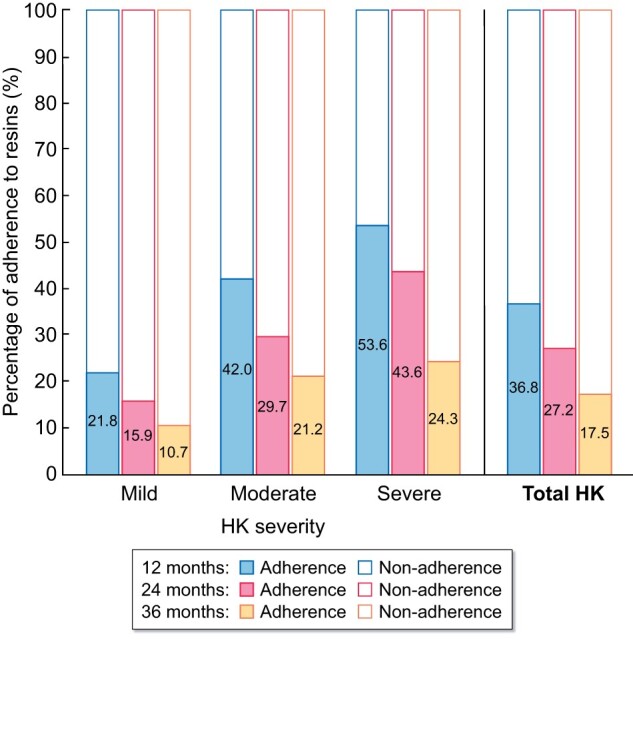
CPS and SPS adherence according to HK severity.
RAASi management and potassium concentration
RAASi treatment was frequently discontinued in all HK groups, with an average discontinuation rate of 43.3%, with higher discontinuation observed in patients treated with resins (51%) (Table 3). Table 4 presents the evolution of the potassium concentration depending on the severity of HK and treatment with ion-exchange resins and RAASis. Most patients with mild and moderate HK had a normal potassium concentration at 36 months, independent of RAASi treatment. However, potassium levels remained elevated in patients with severe HK, even in those treated with ion-exchange resins, despite the fact that potassium reduction was higher in those patients. Severe HK patients on resins decreased to the mild range or lower limit of the moderate range and had a 2- to 7-fold greater reduction in serum potassium than patients with severe HK in whom resins were not prescribed, although patients without resins were very few (Table 4).
Table 3.
Use of RAASis
| Total (N = 1499) | Mild HK (n = 992) | Moderate HK (n = 351) | Severe HK (n = 156) | P-value | |
|---|---|---|---|---|---|
| Total sample (N = 1449) | |||||
| Initial period, n (%) | 1199 (80.0) | 800 (80.6) | 285 (81.2) | 114 (73.1) | |
| After 36 months, n (%) | 680 (45.4) | 482 (48.6) | 143 (40.7) | 55 (35.3) | 0.002 |
| % of RAASi discontinuation | −43.3 | −39.8 | −49.8 | −51.8 | <0.001 |
| P-value* | <0.001 | <0.001 | <0.001 | <0.001 | |
| Patients without CPS or SPS (n = 863) | |||||
| Initial period, n (%) | 698 (72.0) | 561 (79.6) | 127 (89.4) | 10 (62.5) | |
| After 36 months, n (%) | 440 (51.0) | 366 (51.9) | 69 (48.6) | 5 (31.3) | 0.002 |
| % of RAASi discontinuation | −37.0 | −34.8 | −45.7 | −50.0 | <0.001 |
| P-value* | <0.001 | <0.001 | 0.002 | NS | |
| Patients with CPS or SPS (n = 636) | |||||
| Initial period, n (%) | 490 (77.0) | 231 (80.5) | 161 (77.0) | 98 (70.0) | |
| After 36 months, n (%) | 240 (37.7) | 114 (39.7) | 79 (37.8) | 47 (33.6) | 0.002 |
| % of RAASi discontinuation | −51.0 | −50.6 | −50.9 | −52.0 | 0.0871 |
| P-value* | <0.001 | <0.001 | <0.001 | 0.002 | |
NS: not significant; P: statistical significance (difference between mild HK and severe HK).
Statistical significance (difference between initial period and HK after 36 months).
Table 4.
Evolution of average potassium levels according to RAASi use (mEq/L)
| Total (N = 1499) | Mild HK (n = 992) | Moderate HK (n = 351) | Severe HK (n = 156) | P-value | |
|---|---|---|---|---|---|
| n | 1199 | 800 | 285 | 114 | |
| Initial period with RAASi, mean (SD) | 6.0 (0.7) | 5.3 (0.1) | 5.7 (0.2) | 6.8 (0.6) | <0.001 |
| After 36 months (with RAASi), mean (SD) | 5.1 (0.8) | 4.7 (0.7) | 4.9 (0.6) | 5.6 (0.9) | <0.001 |
| Difference | 0.9 | 0.6 | 0.8 | 1.2 | |
| P-value* | <0.001 | <0.001 | <0.001 | <0.001 | |
| n | 300 | 192 | 66 | 42 | |
| Initial period without RAASi, mean (SD) | 6.0 (0.8) | 5.2 (0.1) | 5.7 (0.2) | 6.9 (0.7) | |
| After 36 months (without RAASi), mean (SD) | 5.2 (0.9) | 4.7 (0.7) | 4.9 (0.6) | 5.8 (1.0) | <0.001 |
| Difference | 0.8 | 0.5 | 0.8 | 1.1 | |
| P-value* | <0.001 | <0.001 | <0.001 | <0.001 | |
| Without CPS or SPS (n = 863) | |||||
| n | 698 | 567 | 127 | 4 | |
| Initial period with RAASi, mean (SD) | 5.7 (0.4) | 5.3 (0.1) | 5.7 (0.2) | 6.5 (0.4) | <0.001 |
| After 36 months (with RAASi), mean (SD) | 5.2 (0.7) | 4.9 (0.5) | 5.0 (0.4) | 5.8 (0.8) | <0.001 |
| Difference | 0.5 | 0.4 | 0.7 | 0.7 | |
| P-value* | <0.001 | <0.001 | 0.002 | NS | |
| n | 165 | 147 | 15 | 3 | |
| Initial period without RAASi, mean (SD) | 5.6 (0.4) | 5.2 (0.1) | 5.7 (0.2) | 6.4 (0.2) | |
| After 36 months (without RAASi), mean (SD) | 5.3 (0.8) | 5.0 (0.6) | 5.0 (0.5) | 6.2 (0.9) | <0.001 |
| Difference | 0.3 | 0.2 | 0.7 | 0.2 | |
| P-value* | <0.001 | <0.001 | 0.002 | NS | |
| With CPS or SPS (n = 636) | |||||
| n | 501 | 229 | 158 | 114 | |
| Initial period with RAASi, mean (SD) | 6.3 (0.8) | 5.3 (0.2) | 5.7 (0.2) | 6.9 (0.4) | <0.001 |
| After 36 months (with RAASi), mean (SD) | 4.8 (1.0) | 4.3 (0.7) | 4.5 (0.7) | 5.4 (1.0) | <0.001 |
| Difference | 1.5 | 1.0 | 1.2 | 1.5 | |
| P-value* | <0.001 | <0.001 | <0.001 | 0.002 | |
| n | 135 | 42 | 51 | 42 | |
| Initial period without RAASi, mean (SD) | 6.5 (0.9) | 5.3 (0.1) | 5.7 (0.2) | 7.0 (0.7) | |
| After 36 months (without RAASi), mean (SD) | 5.0 (1.0) | 4.0 (0.7) | 4.6 (0.7) | 5.5 (0.9) | <0.001 |
| Difference | 1.5 | 1.3 | 1.1 | 1.5 | |
| P-value* | <0.001 | <0.001 | <0.001 | 0.002 | |
NS: not significant; P: statistical significance (difference between mild HK and severe HK).
Statistical significance (difference between initial period and HK after 36 months).
Resource use and HK-associated costs
Patients with chronic HK needed a mean of 9.1 hospital visits and 1.3 emergency room visits and were hospitalized for a mean of 3.6 days. Nevertheless, the number of hospitalization days varied according to the severity of HK, ranging from 1.5 days in patients with mild HK to 14 days in patients with severe HK.
Overall, the annual healthcare cost per patient with chronic HK was €5929, with a direct relationship to the severity of HK (Figure 3). Primary care costs accounted for 45.8% and specialized care costs for 54.2% of the overall cost. Of the annual healthcare cost per patient, 31.9% was attributable to HK.
FIGURE 3:

Annual total cost per HK patient.
Concerning specialized care costs, the annual cost per patient was €3213, and again this was directly related to the severity of HK (Figure 4a). In addition, 58.8% of the specialized care costs were attributable to HK. Of these costs, 5.3% were related to hospital emergencies, 8.1% represented the cost of treatment with resins, 24.7% were related to hospital medical visits and 61.9% were associated with hospitalization (Figure 4b).
FIGURE 4:
(A) Annual specialized care cost per HK patient. (B) annual specialized care costs related to HK.
DISCUSSION
This study found that chronic HK in CKD patients with comorbidities in Spain was usually managed with ion-exchange resins and RAASi discontinuation. However, patients were reluctant to maintain resin intake, even those patients with severe HK. The most relevant finding in this study is that HK remained uncontrolled in patients with severe HK and was associated with high numbers of hospitalizations and high healthcare costs. It is also noteworthy that severe HK patients on resins had a 2- to 7-fold greater reduction in serum potassium than patients with severe HK in whom resins were not prescribed.
It has been described that potassium levels >5 mEq/L increase the risk of life-threatening events such as cardiac arrhythmias and sudden death [36]. Therefore, in the present study, we considered HK when potassium was ≥5.1 mEq/L. Patients with CKD show the highest risk of HK, particularly if they are diabetic and treated with RAASi [37]. Accordingly, in this study, most patients were older, diabetic with CKD Stages 2 and 3 and receiving RAASis.
The usual recommendation for patients with CKD and HK is dietary potassium restriction. Unfortunately this information was lacking in this study. However, there is no definitive evidence supporting this recommendation and recent KDIGO guidelines state that although dietary potassium restriction is a valid strategy to treat acute HK, ‘generalized dietary potassium restriction in people with CKD may deprive them from other beneficial effects and nutrient of potassium-rich diets’ [38]. Regarding management of HK in CKD patients with comorbidities, this study confirms previous findings from observational studies [37] describing RAASi cessation as a common practice, and this study found RAASi discontinuation in 43.3% of the entire cohort. RAASis improve kidney and patient survival in CKD, particularly in diabetics and, theoretically, RAASi discontinuation may be associated with worse outcome in this population. Nevertheless, further studies are needed, as this assumption has not yet been proved.
The present study provides interesting data regarding treatment with ion-exchange resins. Indeed, resins were prescribed in 42.5% of patients and in nearly 90% of patients with severe HK. Surprisingly, there is limited high-quality evidence supporting the efficacy of ion-exchange resins in patients with CKD, whereas there are some concerns regarding safety. SPS prescription was associated with increased hospitalization for gastrointestinal adverse events [39, 40], including intestinal necrosis when given with sorbitol [41]. Moreover, ion-exchange resins should be given in patients with normal bowel function and with caution, since they can produce significant gastrointestinal binding of other drugs, limiting their correct absorption [38]. In this study we found a very low adherence to ion-exchange resins, with only 36.6% and 17.5% of adherent patients at 1 and 3 years, respectively. Adherence was not related to the severity of HK. Although, in the present study, the occurrence of these potentially severe adverse effects was not confirmed, our hypothesis is that low adherence is related with low tolerance and the discomfort caused by these medications when given chronically. The non-compliance with resins, or potentially with other therapies, may be due to the slightly increased frequency of depressive symptoms in patients with more severe HK. In addition, this study showed that the percentage of normokalaemia (serum potassium <5.0 mEq/L) was similar in patients regardless of the use of resins, although it was higher in patients without the use of resins.
The management of chronic HK in the real-world population seems to normalize potassium levels in mild and moderate HK at the expense of RAASi discontinuation and still failed to control HK in patients with severe HK. This finding clearly points out that the effective management of HK remains an unmet need. This fact may put this population at the highest risk of morbimortality, and it is associated with increased health expenditures, as we observed in our study and previously reported by others [4, 27]. In our study, moderate and severe HK entailed 3.5 and 8.7 times more risk for hospitalization compared with mild HK and was associated with an overall annual cost of €6993 and €12 705 per patient, respectively, and a significant part of it directly related to HK complications.
Chronic HK in CKD patients with comorbidities can be treated with the new potassium binders patiromer [42] and sodium zirconium cyclosilicate [43, 44]. Studies with 12 months of follow-up provided evidence for efficacy in controlling HK, although they also showed some adverse events [38]. Patiromer has been associated with constipation and hypomagnesaemia and sodium zirconium cyclosilicate with oedema. Moreover, as happens with ion-exchange resins, these new agents interfere with the bioavailability of other medications either by direct binding or by gastric pH alteration. Some studies provide promising data regarding the use of patiromer in patients requiring RAAS blockade, demonstrating both efficacy to control HK and to retain RAASis in up to 94% of patients [45] or 87% of patients [46]. Similar results were reported with sodium zirconium cyclosilicate [47].
This study has some limitations. First, the study only includes patients who required healthcare for an episode of HK and thus generated HK-related healthcare. Second, the study does not provide information regarding dietary potassium restriction, as this information is not available in the data source. Finally, the study was designed to ascertain HK management and costs rather than study CKD progression or mortality. However, this study also has some strengths. First, the real-life healthcare database used contains data of 1.9 million patients in Spain. This database is part of the European Network of Centres for Pharmacoepidemiology and Pharmacovigilance, a network of institutions coordinated by the European Medicines Agency. Second, we were able to follow patients with HK up to 36 months and to precisely analyse the effect of HK on healthcare costs, the use of RAASis, ion-exchange resin adherence and the lack of potassium control in cases of severe HK.
In conclusion, this study points out that there remain unmet needs for chronic HK treatment in patients with comorbidities and CKD. Despite that chronic HK entails a high cost for the National Health System, most patients with severe HK persist uncontrolled and therefore are at risk of HK complications. Moreover, severe HK patients, as well as mild and moderate HK patients, suffer from low adherence to ion-exchange resins and a high proportion of RAASi discontinuation, which may lead to CKD progression and cardiovascular events. Therefore it is advisable to seek new strategies to tackle chronic HK.
FUNDING
This study was funded by Vifor Fresenius Medical Care Renal Pharma España SL, a company of the Vifor Pharma group.
AUTHORS’ CONTRIBUTIONS
M.A., J.D. and J.M.C. participated in the conception or design and analysis and interpretation of data. M.A. and J.M.C. drafted the article. All authors reviewed the draft manuscript, provided intellectual content of critical importance to the work described and read and gave final approval of the version to be published.
CONFLICT OF INTEREST STATEMENT
The results presented in this article have not been published previously in whole or part, except in abstract format. A.O.d.L.L. is affiliated with the Escuela Andaluza de Salud Pública, CIBER en Epidemiología y Salud Pública (CIBERESP) and Instituto de Investigación Biosanitaria. O.D.C. is employed by the Servizo Galego de Saúde. J.M.R.-R. is employed by the Hospital Perpetuo Socorro. M.R.G.D.-G. is employed by the Dirección provincial de Sanidad en Albacete. V.A.P. and M.B.d.l.H.D. are employed by Hospital Nuestra Señora del Prado. M.A. is an employee of BCN Health Economics & Outcomes Research S.L., an independent contract health economic organization. J.D. is employed by the University of Barcelona. J.M.C. is affiliated with the University of Barcelona, Bellvitge Biomedical Research Institute (IDIBELL) and Spanish Network for Renal Research (REDINREN). A.O.d.L.L., O.D., J.M.R.-R., V.A.P., M.B.d.l.H.D. and J.M.C. have received consultancy fees from Vifor Fresenius Medical Care Renal Pharma España S.L. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article apart from those disclosed. BCN Health Economics and Outcomes Research provided statistical analysis and editorial support and their services were funded by Vifor Fresenius Medical Care Renal Pharma España S.L.
Contributor Information
Antonio Olry de Labry Lima, Escuela Andaluza de Salud Pública, Granada, Spain; CIBER en Epidemiología y Salud Pública, Madrid, Spain; Instituto de Investigación Biosanitaria, Hospitales Universitarios de Granada/Universidad de Granada, Granada, Spain.
Óscar Díaz Castro, Servizo de Cardioloxía, Complejo Hospitalario Universitario de Vigo, Servizo Galego de Saúde, Vigo, Pontevedra, Spain.
Jorge M Romero-Requena, Sección de Medicina Interna, Hospital Perpetuo Socorro, Badajoz, Spain.
M de los Reyes García Díaz-Guerra, Inspectora Farmacéutica, Dirección provincial de Sanidad en Albacete, Albacete, Spain.
Virginia Arroyo Pineda, Servicio de Farmacia de Atención Primaria, Hospital Nuestra Señora del Prado, Talavera de la Reina (Toledo), Spain.
M Belén de la Hija Díaz, Servicio de Farmacia de Atención Primaria, Hospital Nuestra Señora del Prado, Talavera de la Reina (Toledo), Spain.
Meritxell Ascanio, BCN Health Economics & Outcomes Research, SL, Barcelona, Spain.
Josep Darbà, Department of Economics, Universitat de Barcelona, Barcelona, Spain.
Josep M Cruzado, Department of Nephrology, Bellvitge University Hospital, L'Hospitalet de Llobregat, Barcelona, Spain; Department of Clinical Sciences, University of Barcelona, Barcelona, Spain; Bellvitge Biomedical Research Institute, L'Hospitalet de Llobregat, Barcelona, Spain; Spanish Network for Renal Research, ISCIII, Madrid, Spain.
REFERENCES
- 1. McMurray JJ, Adamopoulos S, Anker SDet al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012; 33: 1787–1847 [DOI] [PubMed] [Google Scholar]
- 2. Lehnhardt A, Kemper M.. Pathogenesis, diagnosis and management of hyperkalemia. Pediatr Nephrol 2011; 26: 377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Collins AJ, Pitt B, Reaven Net al. Association of serum potassium with all-cause mortality in patients with and without heart failure, chronic kidney disease, and/or diabetes. Am J Nephrol 2017; 46: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Jiménez-Marrero S, Cainzos-Achirica M, Monterde Met al. Impact on clinical outcomes and health costs of deranged potassium levels in patients with chronic cardiovascular, metabolic, and renal conditions. Rev Esp Cardiol (Engl Ed) 2021; 74: 312–320 [DOI] [PubMed] [Google Scholar]
- 5. Savarese G, Xu H, Trevisan Met al. Incidence, predictors, and outcome associations of dyskalemia in heart failure with preserved, mid-range, and reduced ejection fraction. JACC Heart Fail 2019; 7: 65–76 [DOI] [PubMed] [Google Scholar]
- 6. Thomsen RW, Nicolaisen SK, Hasvold Pet al. Elevated potassium levels in patients with congestive heart failure: occurrence, risk factors, and clinical outcomes: a Danish population-based cohort study. J Am Heart Assoc 2018; 7: e008912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Morales E, Torregrosa JV.. Hiperpotasemia crónica o persistente, una vieja entidad con nuevos protagonistas. Nefrología 2019; 1: 1–49 [Google Scholar]
- 8. Crespo-Leiro MG, Barge-Caballero E, Segovia-Cubero Jet al. Hiperpotasemia en pacientes con insuficiencia cardiaca en España y su impacto en las recomendaciones. Registro ESC-EORP-HFA Heart Failure Long-Term. Rev Esp Cardiol 2020; 73: 313–32331672562 [Google Scholar]
- 9. Valdivielso J, Betriu A, Bermudez Lopez Met al. Prevalencia de hiperpotasemia, factores asociados y efecto sobre la morbimortalidad cardiovascular. Nefrología 2017; 37: 48 [Google Scholar]
- 10. Kovesdy CP. Management of hyperkalemia: an update for the internist. Am J Med 2015; 128: 1281–1287 [DOI] [PubMed] [Google Scholar]
- 11. Asensio MMJ, Herrero D. L E, Estébanez MBet al. Alteraciones del potasio. Medicine 2015; 11: 4739–4747 [Google Scholar]
- 12. Ponikowski P, Voors AA, Anker SDet al. ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129–2200. [DOI] [PubMed] [Google Scholar]
- 13. National Kidney Foundation. Guideline 11: use of angiotensin-converting enzyme inhibitors and angiotensin receptor blockers in CKD. In: K/DOQI Clinical Practice Guidelines on Hypertension and Antihypertensive Agents in Chronic Kidney Disease. 2002. http://www2.kidney.org/professionals/kdoqi/guidelines_bp/guide_11.htm (February 2015, date last accessed)
- 14. Kidney Disease: Improving Global Outcomes CKD Work Group. KDIGO 2012 clinical practice guideline for the evaluation and management of chronic kidney disease. Kidney Int Suppl 2013; 3: 1–150 [DOI] [PubMed] [Google Scholar]
- 15. Yancy CW, Jessup M, Bozkurt Bet al. ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810–1852. [DOI] [PubMed] [Google Scholar]
- 16. Lindenfeld JA, Albert NM, Boehmer JPet al. HFSA 2010 comprehensive heart failure practice guideline. J Card Fail 2010; 16: e1–194 [DOI] [PubMed] [Google Scholar]
- 17. Dunn JD, Benton WW, Orozco-Torrentera Eet al. The burden of hyperkalemia in patients with cardiovascular and renal disease. Am J Manag Care 2015; 21(15 Suppl): s307–15 [PubMed] [Google Scholar]
- 18. Buse JB, Wexler DJ, Tsapas Aet al. 2019 update to: management of hyperglycemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care 2020; 43: 487–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt Bet al. ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017; 136: e137–e161. [DOI] [PubMed] [Google Scholar]
- 20. Cosentino F, Grant PJ, Aboyans Vet al. ESC guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD. Eur Heart J 2020; 41: 255–323. [DOI] [PubMed] [Google Scholar]
- 21. National Kidney Foundation. K/DOQI clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. 2004. kidneyfoundation.cachefly.net/professionals/KDOQI/guidelines_bp/index.htm (July 2020, last date accessed)
- 22. National Institute for Health and Care Excellence. Chronic kidney disease in adults: assessment and management. 2014. (updated 2015). nice.org.uk/CG182 (15 July 2020, date last accessed) [PubMed]
- 23. Gorriz JL, Muijsemberg A, Gimenez-Civera Eet al. Characteristics of the population receiving treatment with calcium polystyrene sulfonate for hypercalemia. Low rate of compliance and collection of prescriptions in the pharmacy office. ERA-EDTA, Budapest, European Renal Association–European Dialysis and Transplant Association (ERA–EDTA), 56th Annual Congress, 13–16 June 2019, Budapest, Hungary
- 24. Perazella MA. Drug-induced hyperkalemia: old culprits and new offenders. Am J Med 2000; 109: 307–314 [DOI] [PubMed] [Google Scholar]
- 25. Ouwerkerk W, Voors AA, Anker SDet al. Determinants and clinical outcome of uptitration of ACE-inhibitors and beta-blockers in patients with heart failure: a prospective European study. Eur Heart J 2017; 38: 1883–1890 [DOI] [PubMed] [Google Scholar]
- 26. Epstein M, Reaven NL, Funk SEet al. Evaluation of the treatment gap between clinical guidelines and the utilization of renin-angiotensin-aldosterone system inhibitors. Am J Manag Care 2015; 21(11 Suppl): S212–S220 [PubMed] [Google Scholar]
- 27. Fitch K, Woolley JM, Engel Tet al. The clinical and economic burden of hyperkalemia on medicare and commercial payers. Am Health Drug Benefits 2017; 10: 202–210 [PMC free article] [PubMed] [Google Scholar]
- 28. Real Life Data. 2011. http://www.rlifedata.com/index.php/quienes-somos/ (20 May 2020, date last accessed)
- 29. Charlson ME, Pompei P, Ales KLet al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 1987; 40: 373–383 [DOI] [PubMed] [Google Scholar]
- 30. Charlson ME, Charlson RE, Peterson JCet al. The Charlson comorbidity index is adapted to predict costs of chronic disease in primary care patients. J Clin Epidemiol 2008; 61: 1234–1240 [DOI] [PubMed] [Google Scholar]
- 31. Hurst JW. The value of using the entire New York Heart Association's classification of heart and vascular disease. Clin Cardiol 2006; 29: 415–417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dilla T, Valladares A, Lizán Let al. Adherencia y persistencia terapéutica: causas, consecuencias y estrategias de mejora. Aten Primaria 2009; 41: 342–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Spanish Ministry of Health. Regional tariff lists. https://www.mscbs.gob.es/estadEstudios/estadisticas/inforRecopilaciones/anaDesarrolloGDRanteriores.htm (20 May 2020, date last accessed)
- 34. INE. https://www.ine.es/ ( 20 May 2020, date last accessed)
- 35. General Council of Official Colleges of Pharmacists. Bot Plus database. BOT Plus 2. https://botplusweb.portalfarma.com/ (20 May 2020, last date accessed)
- 36. Epstein M, Pitt B.. Recent advances in pharmacological treatments of hyperkalemia: focus on patiromer. Expert Opin Pharmacother 2016; 17: 1435–1448 [DOI] [PubMed] [Google Scholar]
- 37. Wetmore JB, Yan H, Horne Let al. Risk of hyperkalemia from renin-angiotensin-aldosterone system inhibitors and factors associated with treatment discontinuities in a real-world population. Nephrol Dial Transplant 2019; doi: 10.1093/ndt/gfz263 [DOI] [PubMed] [Google Scholar]
- 38. Clase CM, Carrero JJ, Ellison DHet al. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int 2020; 97: 42–61. [DOI] [PubMed] [Google Scholar]
- 39. Noel JA, Bota SE, Petrcich Wet al. Risk of hospitalization for serious adverse gastrointestinal events associated with sodium polystyrene sulfonate use in patients of advanced age. JAMA Intern Med 2019; 179: 1025–1033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Laureati P, Xu Y, Trevisan Met al. Initiation of sodium polystyrene sulphonate and the risk of gastrointestinal adverse events in advanced chronic kidney disease: a nationwide study. Nephrol Dial Transplant 2020; 35: 1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Sterns RH, Rojas M, Bernstein Pet al. Ion-exchange resins for the treatment of hyperkalemia: are they safe and effective? J Am Soc Nephrol 2010; 21: 733–735 [DOI] [PubMed] [Google Scholar]
- 42. Colbert GB, Patel D, Lerma EV.. Patiromer for the treatment of hyperkalemia. Expert Rev Clin Pharmacol 2020; 13: 563–570 [DOI] [PubMed] [Google Scholar]
- 43. Fishbane S, Ford M, Fukagawa Met al. A phase 3b, randomized, double-blind, placebo-controlled study of sodium zirconium cyclosilicate for reducing the incidence of predialysis hyperkalemia. J Am Soc Nephrol 2019; 30: 1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Packham DK, Rasmussen HS, Singh B.. New agents for hyperkalemia. N Engl J Med 2015; 372: 1571–1572 [DOI] [PubMed] [Google Scholar]
- 45. Weir MR, Bakris GL, Bushinsky DAet al. Patiromer in patients with kidney disease and hyperkalemia receiving RAAS inhibitors. N Engl J Med 2015; 372: 211–221 [DOI] [PubMed] [Google Scholar]
- 46. Agarwal R, Rossignol P, Romero Aet al. Patiromer versus placebo to enable spironolactone use in patients with resistant hypertension and chronic kidney disease (AMBER): a phase 2, randomised, double-blind, placebo-controlled trial. Lancet 2019; 394: 1540–1550 [DOI] [PubMed] [Google Scholar]
- 47. Spinowitz BS, Fishbane S, Pergola PEet al. Sodium zirconium cyclosilicate among individuals with hyperkalemia: a 12-month phase 3 study. Clin J Am Soc Nephrol 2019; 14: 798–809. [DOI] [PMC free article] [PubMed] [Google Scholar]




