FIG 5.
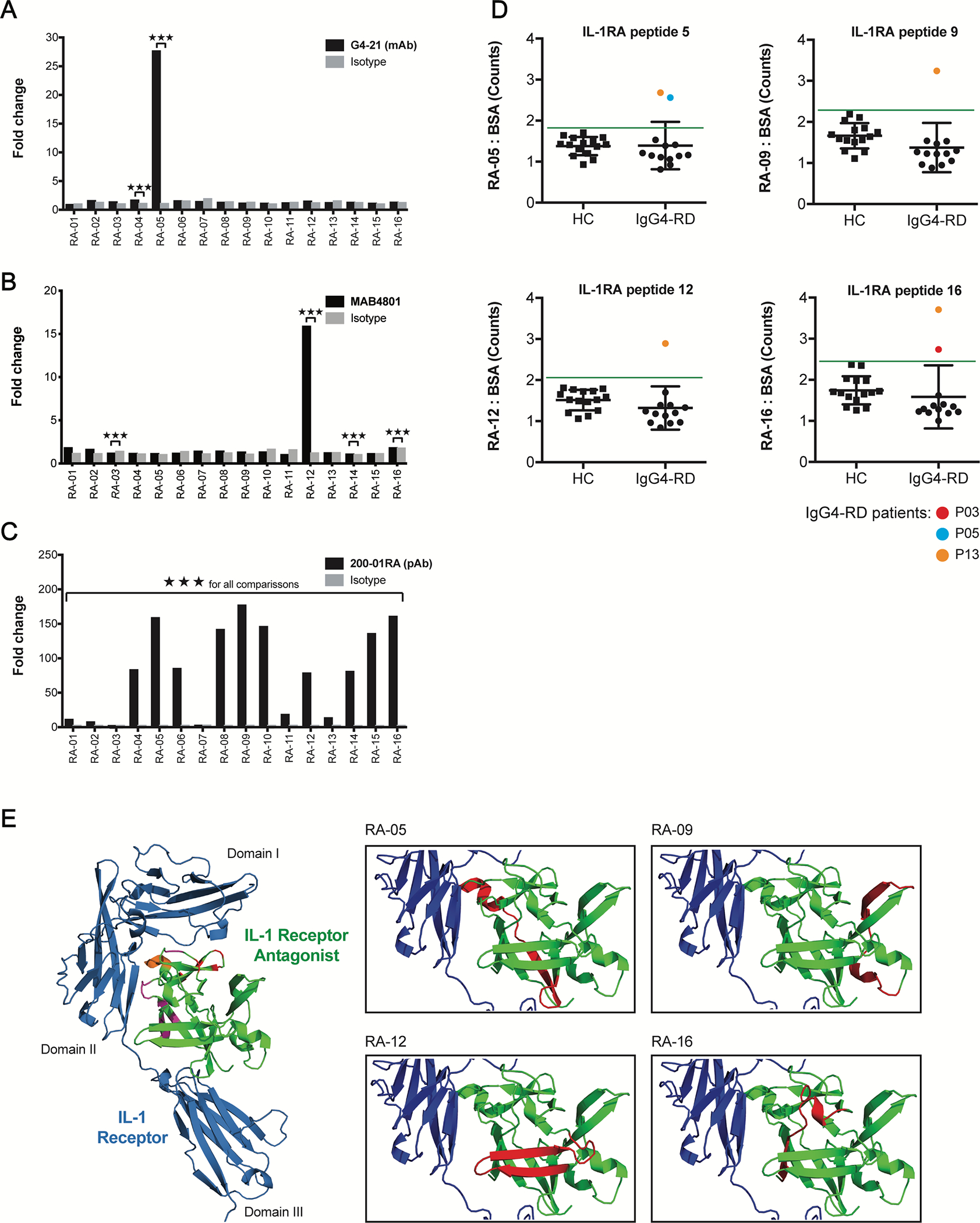
Anti-IL-1RA antibodies target IL-1RA peptides near the IL-1 Receptor interface. A – C, ELISA quantification of the IgG4-RD patient-derived mAb, G4–21 (A), commercial anti-IL-1RA mAb, MAB4801 (B), and commercial anti-IL-1RA pAb, 200–01RA (C), binding to sixteen 19-mer overlapping human IL-1RA peptides. Fold change represents europium counts of each peptide over the minimum peptide reactivity (across all peptides) per antibody. D, ELISA quantification of IgG binding to four immunogenic IL-1RA peptides identified in A – C in the plasma of patients with IgG4-RD (IgG4-RD; n = 13) and healthy controls (HC; n 15). Data represent mean ± SD of europium counts of triplicate wells. Green line indicates threshold for IL-1RA peptide binding (3 SD above the mean europium count from the healthy control group). Colored data points indicate IgG4-RD patient samples positive for autoantibodies for a given peptide. E, 3D structure of human IL-1 Receptor (blue) in complex with human IL-1RA (green; PDB#1IRA). Left: IL-1RA residues implicated in binding IL-1 Receptor at domains I and II and at their interface are highlighted in red, purple, and orange, respectively. Right: Peptides 05, −09, −12, and −16 targeted by anti-IL-1RA autoantibodies are highlighted in red. ***P<0.001 by two-tailed unpaired Student’s t tests.
