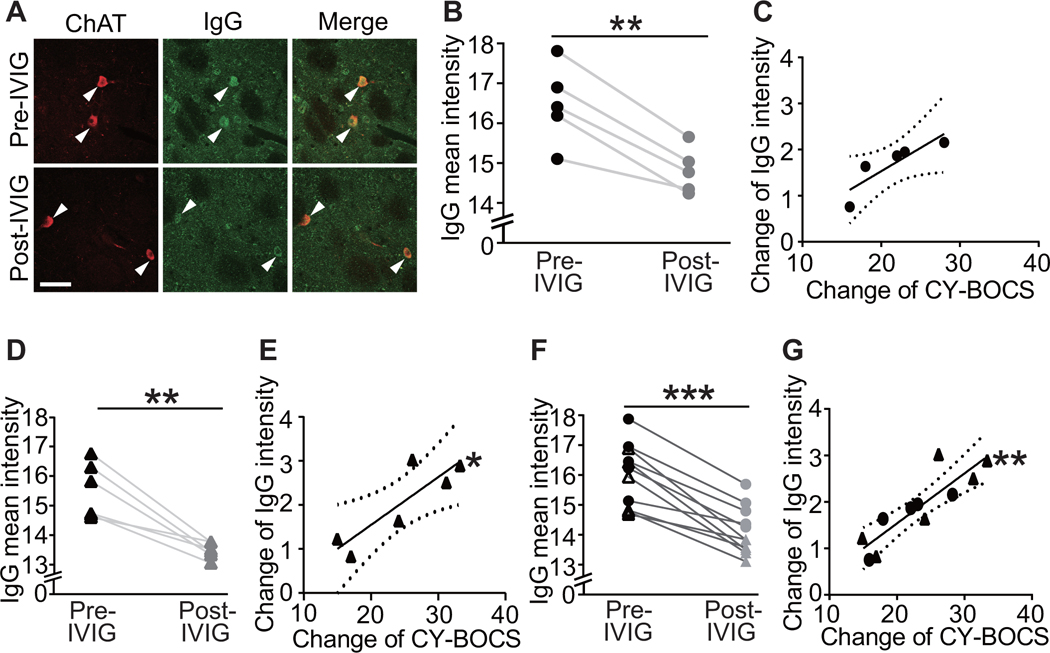
Figure 4. Reduction in PANDAS IgG binding to cholinergic interneurons correlates with decreases in CY-BOCS score following IVIG treatment.
Sera were collected at baseline (pre-IVIG) and 6–12 weeks after
intravenous immunoglobulin (IVIG) treatment (post-IVIG) and tested for CIN
binding. (A) Representative images of co-staining of anti-human IgG (green) and
anti-ChAT (red) before and after IVIG treatment. Arrowheads indicate antibody
binding to CINs. Scale bar represents 40 μm. (B) IVIG treatment results
in decreased PANDAS IgG binding to CINs in the original 5 sera (paired t-test:
t[4]=6.870, p=0.002); this constitutes a technical replication of our previous
analysis of the same samples, using a different assay (47). (C) The change in IgG binding to striatal CINs
correlated at trend level with symptom improvement (Pearson’s
correlation: r2=0.753, p=0.057). (D) IVIG treatment similarly
produced decreased PANDAS IgG binding to CINs in the second cohort (t[5]=5.413,
p=0.003). (E) Change in IgG binding to striatal CINs correlated significantly
with symptom improvement in the second cohort (r2=0.747, p=0.026).
(F) The consistent effect of IVIG treatment across the two cohorts was
particularly apparent in pooled data (t[10]=8.191, p<0.0001) (G) In
pooled data, the correlation between change in serum IgG binding to CINS and
improvement in symptoms was particularly robust (r2=0.762, p=0.0005).
Each data point represents mean value obtained from 4–6 mice for one
serum. **p<0.01, ***p<0.001; N=5 in B and C, N=6 in D and E, N=11
in F and G, for each group. Dotted lines in correlation analyses indicate 95%
confidence intervals. ●, – 5 serum pairs from the first cohort (47); ▲,
– 5 serum pairs from the first cohort (47); ▲, – 6 serum pairs from the second cohort.
– 6 serum pairs from the second cohort.