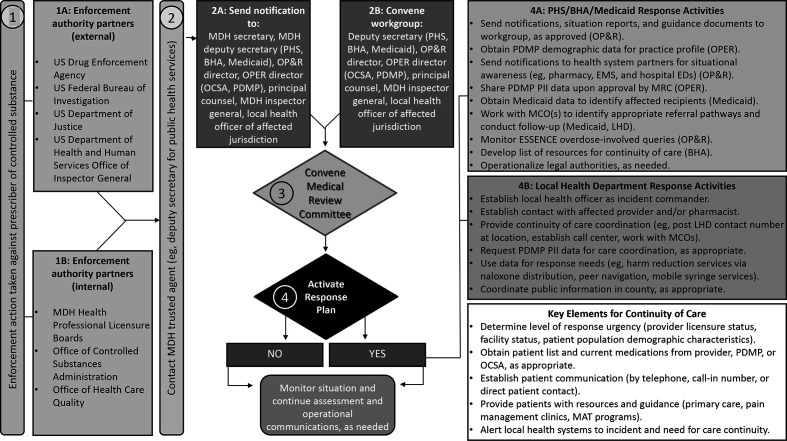
Figure.
Maryland Department of Health (MDH) Controlled Substances Interagency Response Workgroup enforcement action decision flow chart. In step 1, action is taken against a prescriber from an external or internal enforcement body (ie, any federal, state, or local enforcement authority or other governmental investigative authority; 1A, 1B). In step 2, the MDH trusted agent (the deputy secretary of public health services and/or the OP&R director) is contacted and notified of imminent sanction. The MDH trusted agent alerts executive leadership (2A) and convenes Workgroup members (2B). Workgroup members are directed to gather information and data on where patients reside and receive treatment in preparation for the meeting. In step 3, the Workgroup is convened (2B) to discuss the facts as stated in the enforcement action notification and decide next steps for convening a medical review committee (MRC). The prescriber cannot be identified until the order is public. In step 4, the MRC reviews MDH data to decide whether to activate a response plan. If yes, any of the actions in boxes 4A and 4B may be taken. For example, the state may customize template notifications for local partners about the situation (eg, local hospitals and emergency departments [EDs], pharmacies, local law enforcement, EMS). If not, the MRC will monitor the situation and provide situational updates to leadership. Abbreviations: BHA, Behavioral Health Administration; CS, controlled substance; EMS, emergency medical services; ESSENCE, Early Notification of Community-based Epidemics; LHD, local health department; MAT, medication assisted treatment; MCO, managed care organization; OCSA, Office of Controlled Substances Administration; OP&R, Office of Preparedness and Response; OPER, Office of Provider Engagement and Regulation; PDMP, prescription drug monitoring program; PHS, Public Health Services; PII, personally identifiable information.