Abstract
The CONCEPTT trial compared real-time Continuous Glucose Monitoring (RT-CGM) to capillary glucose monitoring in pregnant women with type 1 diabetes. We analyzed CGM and glycated hemoglobin (HbA1c) measures in first (n = 221), second (n = 197), and third (n = 172) trimesters, aiming to examine target glucose attainment and associations with pregnancy outcomes. CGM targets were Time-in-range (TIR) > 70%, Time-above-range (TAR) <25%, and Time-below-range (TBR) < 4%, and HbA1c targets < 6.5% (National Institute for Health and Care Excellence [NICE]) and HbA1c < 6.0% in second and third trimesters (American Diabetes Association [ADA]). TIR/TAR/TBR targets were achieved by 7.7/14.5/30.3% participants in first, 10.2/14.2/52.8% in second, and 35.5/37.2/52.9% in third trimesters. CGM target attainment was low but increased during pregnancy and with RT-CGM use. In the adjusted analyses, achieving TBR target was associated with a higher risk of pre-eclampsia and neonatal hypoglycemia. ADA HbA1c target attainment was low and unchanged during pregnancy (23.5/27.9/23.8%) but increased with RT-CGM use. In the adjusted analyses, HbA1c target attainment was associated with a lower risk of preterm birth, large-for-gestational age and neonatal hypoglycemia. We conclude that CONCEPTT trial participants had a low rate of CGM and of HbA1c target attainment. Attainment of CGM and NICE HbA1c targets increased throughout gestation and all targets (both NICE/ADA HbA1c and CGM) were more likely to be achieved by RT-CGM users, at 34 weeks' gestation. ADA HbA1c target achievement was independently associated with better perinatal outcomes, while the independent association of TBR target achievement with increased risk warrants further study. ClinicalTrials.gov Registration Identifier NCT01788527.
Keywords: Continuous glucose monitoring, Type 1 diabetes, Pregnancy, HbA1c target, Time-in-range
Introduction
Standardized continuous glucose monitoring (CGM) metrics and glycated hemoglobin (HbA1c) are recommended for monitoring glucose in people with diabetes.1 The international consensus on Time-in-range (TIR) for CGM data interpretation was published in 2019.2 The recommended percentages of glucose readings in the target range for pregnant women with type 1 diabetes mellitus (T1D) were >70% time (16 h, 48 min) for TIR of 3.5–7.8 mmol/L (63–140 mg/dL), <25% time (6 h) for Time-above-range (TAR) of >7.8 mmol/L (>140 mg/dL), and <4% time (1 h) for Time-below-range (TBR) of <3.5 mmol/L (<63 mg/dL). These values were based on the CONCEPTT trial3 and a cohort study.4
CONCEPTT was a randomized controlled trial, which included 215 pregnant women and 110 women planning pregnancy, assigned to real-time CGM (RT-CGM) in addition to capillary glucose monitoring or capillary glucose monitoring alone (plus 6-day masked CGM in early, mid, and late gestation).3 Pregnant RT-CGM users had improved glucose control (HbA1c, TIR, and TAR at 34 weeks) and pregnancy outcomes (infants large-for-gestational age [LGA], with neonatal hypoglycemia requiring intravenous dextrose or admission to neonatal intensive care unit [NICU] longer than 24 h). The cohort study included 186 pregnant women with T1D using RT-CGM or intermittent monitoring.4 In both studies, a higher TIR was associated with a reduced risk of LGA. Each 5% increase in TIR was associated with benefits for neonatal outcomes.5
In turn, HbA1c is well recognized as the traditional gold standard of glycemic control and biomarker of maternal and neonatal complications.6 Nevertheless, HbA1c has limitations outside and during pregnancy, since its results can be affected by factors such as ethnicity,1,7 age,8 and erythrocyte disorders.9–11 HbA1c targets for pregnant women with T1D are <6.5% (48 mmol/mol) before and throughout pregnancy as recommended by the National Institute for Health and Care Excellence (NICE)12 and <6.5% (48 mmol/mol) before pregnancy/first trimester and <6.0% (42 mmol/mol) in second and third trimesters as recommended by the American Diabetes Association (ADA).6 Although we have studied TIR and HbA1c in this population, we have not addressed attaining the recommended goals.13
The main objective of this subanalysis was to examine CGM-based and ADA HbA1c target glucose attainment in pregnant women participating in the CONCEPTT trial. As a secondary objective, we aimed to evaluate the associations between CGM and HbA1c target attainment with pregnancy outcomes.
Materials and Methods
All centers received ethical approval. All participants gave written informed consent. The current study derives from a pre-specified secondary analysis approved by the CONCEPTT trial steering committee before trial completion.
CGM measurements and blood sampling were performed as detailed in the study protocol.14 We analyzed 6-day CGM readings (Guardian REAL-Time or MiniMed Minilink System, Medtronic, Northridge, CA in the intervention group and masked iPro2 Professional CGM, Medtronic, Northridge, CA, USA, in the control group) at first trimester, 24, and 34 weeks' gestation. HbA1c measures were taken at the same three time points. After delivery, HbA1c samples were measured in the central laboratory (DynaCare, Brampton, ON, Canada) using the turbidimetric inhibition immunoassay for hemolyzed whole blood on the Cobas Integra 700 platform (Roche, Basel, Switzerland).3 Women were included if both CGM and HbA1c data were available at the study time points. The primary outcome addressed was percentage of women achieving the proposed CGM (TIR, TAR, and TBR) and HbA1c targets as already defined, at the three time points. The outcomes tested versus CGM and HbA1c target attainment were pre-eclampsia, cesarean section, preterm birth, LGA, neonatal hypoglycemia, and NICU admission.
We performed descriptive statistics to characterize the group and chi-square and nonparametric tests for bivariate comparisons (Mann–Whitney/Kruskal–Wallis tests). Logistic regression analysis was used to estimate the ability of CGM and HbA1c target attainment in each trimester to predict pregnancy outcomes. A second set of models weas adjusted by age, body mass index (BMI), duration of T1D, ethnicity, parity, center, randomization arm, preconception planning, and smoking habit. No imputations were used. Significance was set at P < 0.05. Analyses were performed using IBM SPSS Statistics for Windows, Version 26.0 (IBM Corp., Armonk, NY, USA).
Results
The potential study population was 249 women, the 215 participants enrolled in the pregnancy trial and the 34 women in the planning pregnancy trial who became pregnant. Women included in this subanalysis were 221 at baseline, 197 at 24 weeks, and 172 at 34 weeks; corresponding figures for maternal and neonatal outcomes for women delivering a live birth at ≥20 weeks were 204, 196, and 171. The characteristics of included women were mean age 31.5 years, BMI 25.8 kg/m2, T1D duration 16.8 years, gestational age at randomization/pregnancy confirmation 10.2 weeks and baseline HbA1c 6.9% (52 mmol/mol) (Supplementary Table S1, very similar to all pregnant CONCEPTT participants).
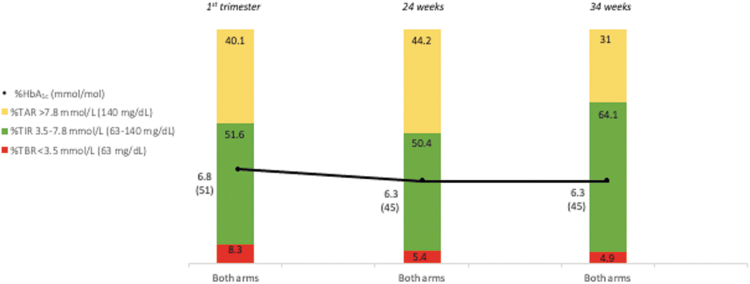
Figure 1 displays average TIR, TAR, TBR, and HbA1c in each trimester, P-values for change over time were P < 0.001 for all metrics. Table 1 gives the proportion of women who reached the recommended CGM-based and HbA1c targets in each trimester. Overall, the rate of CGM target attainment increased during pregnancy. At 34 weeks, the percentage of women achieving TIR, TAR, and TBR targets was higher in the RT-CGM group than in the control group. The percentage of women fulfilling none of the CGM targets was 53.8% at baseline, 32% at 24 weeks, and 5.8% at 34 weeks. The simultaneous attainment of the three CGM targets was 2.7%, 2%, and 17% respectively.
FIG. 1.
CGM-based TIR and HbA1c in pregnant women included in the subanalysis. The diagram displays average TIR, TAR, TBR, and HbA1c in each trimester for RT-CGM and control arms combined. P-values for change over time were P < 0.001 for TAR, P < 0.001 for TIR, P < 0.001 for TBR, and P < 0.001 for HbA1c. CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; RT-CGM, real-time CGM; TAR, Time-above-range; TBR, Time-below-range; TIR, Time-in-range. Color graphics appear online.
Table 1.
Continuous Glucose Monitoring AND Glycated Hemoglobin Target Attainment During Type 1 Diabetes Pregnancy
| Type 1 diabetes pregnancy glucose targets |
Percentage of women fulfilling glucose targets |
P-value for change over time for both arms for RT-CGM, for control arm | ||
|---|---|---|---|---|
| First trimester (N = 221) |
24 Weeks (N = 197) |
34 Weeks (N = 172) |
||
| Both arms |
Both arms |
Both arms |
||
| RT-CGM versus control | RT-CGM versus control | RT-CGM versus control | ||
| CGM | ||||
| TIR 3.5–7.8 mmol/L (63–140 mg/dL) >70% | 7.7 | 10.2 | 35.5 | <0.001 |
| 6.2 versus 9.3ns | 10.5 versus 9.8ns | 44.0 versus 27.3a | <0.001, <0.001 | |
| TAR >7.8 mmol/L (>140 mg/dL) <25% | 14.5 | 14.2 | 37.2 | <0.001 |
| 15.9 versus 13ns | 16.8 versus 11.8ns | 46.4 versus 28.4a | <0.001, <0.01 | |
| TBR <3.5 mmol/L (<63 mg/dL) <4% | 30.3 | 52.8 | 52.9 | <0.001 |
| 26.5 versus 34.3ns | 53.7 versus 52ns | 63.1 versus 43.2b | <0.001, <0.05 | |
| Laboratory | ||||
| ADA trimester-specific HbA1c target | 23.5 | 27.9 | 23.8 | ns |
| 23.0 versus 24.1ns | 30.5 versus 25.5ns | 31.0 versus 17.0a | ns, ns | |
| NICE HbA1c target <6.5% (48 mmol/mol) | 23.5 | 59.4 | 54.1 | <0.001 |
| 23.0 versus 24.1ns | 65.3 versus 53.9ns | 63.1 versus 45.5a | <0.001, <0.001 | |
| HbA1c <6.0% (42 mmol/mol) | 3.6 | 27.9 | 23.8 | <0.001 |
| 2.7 versus 4.6ns | 30.5 versus 25.5ns | 31.0 versus 17.0a | <0.001, <0.001 | |
ADA indicates HbA1c <6.5% (48 mmol/mol) as prepregnancy target and <6.0% (42 mmol/mol) in the second and third trimesters.
P value RT-CGM versus control: a<0.05; b<0.01.
ADA, American Diabetes Association; CGM, continuous glucose monitoring; HbA1c, glycated hemoglobin; NICE, National Institute for Health and Care Excellence; NS, nonsignificant; RT-CGM, real-time CGM; TAR, Time-above-range; TBR, Time-below-range; TIR, Time-in-range.
CGM metrics according to HbA1c target attainment at the three time points were for HbA1c <6.5%: TIR 60%/57%/69.3%, TAR 31%/37.3%/25.3%, and TBR 7%/5%/4%, and for HbA1c <6%: TIR 67.3%/61.4%/71.9%, TAR 16%/32%/22.6%, and TBR 7%/5.2%/4.2%.
Table 1 also gives the rates of women attaining HbA1c targets in each trimester (ADA period-specific goals, <6.5% [48 mmol/mol] and <6.0% [42 mmol/mol]). The percentage of women with HbA1c <6.5% (48 mmol/mol) and <6.0% (42 mmol/mol) increased during pregnancy but the proportion of those achieving period-specific goals did not change. However, RT-CGM and control groups differed at 34 weeks in the frequency of HbA1c target attainment (31% vs. 17%, P = 0.032) due to a nonsignificant increase in RT-CGM group and a nonsignificant decrease in the control group.
Pregnancy outcomes were as follows: 13.1% pre-eclampsia, 67% cesarean section, 40.3% preterm birth, 61.3% LGA, 24.5% neonatal hypoglycemia, and 37.3% NICU admission.
Associations for target attainment with pregnancy outcomes are displayed in Supplementary Table S2. Achieving the TIR target at 34 weeks was associated with a lower risk of preterm birth, achieving the TAR target at 24 weeks was associated with a lower risk of LGA, and achieving the TAR target at 34 weeks was associated with a lower risk of both LGA and preterm birth. In contrast, achieving the TBR target at 24 weeks was associated with an increased risk of neonatal hypoglycemia and NICU admission. Regarding HbA1c, a value <6.5% (48 mmol/mol) in the first trimester was associated with a lower risk of LGA. An HbA1c < 6.0% (42 mmol/mol) at 24 weeks was associated with a lower risk of preterm birth, LGA, neonatal hypoglycemia, and NICU admission; an HbA1c < 6.0% (42 mmol/mol) at 34 weeks was associated with a lower risk of preterm birth, LGA and neonatal hypoglycemia.
After adjustment for clinical variables, attaining TBR target in the first trimester was associated with an increased risk of pre-eclampsia, and with increased risk of neonatal hypoglycemia at 24 weeks. For HbA1c, attaining ADA trimester-specific target in first trimester was associated with a reduced risk of LGA, with reduced risk of both preterm birth and neonatal hypoglycemia at 24 weeks, and preterm birth at 34 weeks.
Discussion
In this subanalysis, we observed that CONCEPTT trial participants had a low rate of CGM TIR target attainment that increased during pregnancy and peaked at 44% of women in the RT-CGM group at 34 weeks. Similarly, rates of HbA1c below the ADA cutoff for each trimester were achieved in less than one-third of women, were unchanged during pregnancy, and highest in the RT-CGM group at 34 weeks.
In the unadjusted analyses, achieving TIR and especially TAR targets at 24 and 34 weeks was associated with better perinatal outcomes in terms of preterm birth and LGA. However, TBR target attainment was associated with a higher risk of neonatal hypoglycemia and NICU admission. Achieving ADA HbA1c trimester-specific targets was associated with better neonatal outcomes in all trimesters (LGA in the first, four outcomes at 24 weeks, and three outcomes at 34 weeks).
In the adjusted analyses, achieving ADA HbA1c trimester-specific targets was independently associated with better perinatal outcomes. However, for CGM targets, the only significant associations were for increased risk in association with attainment of TBR target. This could be attributed to the fact that most women achieving <4% TBR did not achieve TIR or TAR targets, but adjusted odds ratios for TIR and TAR do not support this interpretation. An alternative hypothesis is that spending more time at low glucose values is associated with better neonatal outcomes. The lower TBR cutoff in pregnant women is due to glucose levels being physiologically lower during pregnancy. In fact, data from healthy pregnant women indicate that rates of glucose readings <3.5 mmol/L (<63 mg/dL) are higher than 4%.15,16 The association between TBR targets and pregnancy outcomes warrants further study with newer generation CGM sensors with improved accuracy in the lower glucose range. In addition to carefully balancing the maternal risks of severe hypoglycemia in women with T1D with potential neonatal benefits, modification of current TBR cutoffs would require tools to safely bring glucose to low values.
A strength of these results is that they provide evidence from a well-designed multicenter international trial. Notably, to our knowledge, this subanalysis is the first to compare the attainment of CGM-based targets using TIR International Consensus Report with HbA1c targets in pregnant women with T1D. However, some limitations are worth noting. First, we only analyzed 6-day CGM readings; associations of CGM metrics with perinatal outcomes could have been more robust if they had been measured throughout pregnancy, but this would have limited the analysis to the RT-CGM group. Second, as trial criteria for pregnancy enrolment excluded women with first pregnancy HbA1c <6.5% (48 mmol/mol) or at enrolment >10.0% (86 mmol/mol), our observations do not include the whole spectrum of glycemia in early pregnancy.14
The rates of CGM target attainment were low and despite their increase throughout gestation, peak targets were only achieved by 44% of women for TIR, 46.4% for TAR, and 63.1% for TBR at 34 weeks in the RT-CGM group. Similarly, rates of HbA1c below the ADA cutoff for each trimester were achieved in less than one third of women, and even when they did not increase significantly during pregnancy, the rate of HbA1c <6.0% (42 mmol/mol) was higher at 34 weeks in the RT-CGM group. According to the proportion of women attaining different targets, TIR and TAR targets were more stringent than HbA1c <6.5% (48 mmol/mol) throughout pregnancy, whereas for HbA1c <6.0% (42 mmol/mol), it depended on the trimester. ADA HbA1c target achievement was superior to 6-day CGM measures in the prediction of pregnancy outcomes, reflecting the fact that HbA1c is a measure of average glucose over a 2–3-month period.17 Other studies have addressed CGM metrics and/or HbA1c in pregnant women with pregestational diabetes but not target attainment for both biomarkers.18–21
In clinical practice, to minimize complications attributable to fetal hyperinsulinism, efforts aim at achieving and sustaining maternal glucose in the target range throughout pregnancy. Our results suggest that, even when RT-CGM has been shown to improve clinical outcomes, additional improvement is required for women to reach the tight CGM TIR and ADA HbA1c targets before late gestation. Possible solutions may include better pre-pregnancy planning, lifestyle changes, as well as treatment and technological advances. Preliminary data suggest that interventions such as closed-loop systems may be beneficial for supporting pregnant women with T1D to safely achieve higher TIR and lower TAR.22,23
Conclusions
In conclusion, CONCEPTT trial participants had a low rate of CGM and of HbA1c target attainment especially for the trimester-specific ADA HbA1c targets, which were unchanged during pregnancy. Attainment of CGM and NICE HbA1c targets increased throughout gestation and all targets (both NICE/ADA HbA1c and CGM) were more likely to be achieved by RT-CGM users, at 34 weeks' gestation. ADA HbA1c target achievement was independently associated with better perinatal outcomes, whereas the independent association of TBR target achievement with increased risk warrants further study.
Supplementary Material
Acknowledgments
The authors thank all women with T1D who participated. We also acknowledge the invaluable support from the 31 clinical care teams and the CONCEPTT Steering Committee: Denice S. Feig, Helen R. Murphy, Elisabeth Asztalos, Jon F.R. Barrett, Rosa Corcoy, Alberto de Leiva, Lois E. Donovan, J. Moshe Hod, Lois Jovanovic,† Erin Keely, Craig Kollman, Ruth McManus, Kellie E. Murphy, Katrina Ruedy, Marlon Pragnell, Olivia Lou, Aaron Kowalski, and George Tomlinson.
Contributor Information
On Behalf of the CONCEPTT Collaborative Group:
Helen Murphy, Jeannie Grisoni, Carolyn Byrne, Sandra Neoh, Katy Davenport, Lois Donovan, Claire Gougeon, Carolyn Oldford, Catherine Young, Stephanie Amiel, Katharine Hunt, Louisa Green, Helen Rogers, Benedetta Rossi, Denice Feig, Barbara Cleave, Michelle Strom, Alberto de Leiva, Juan María Adelantado, Ana Isabel Chico, Erin Keely, Janine Malcolm, Kathy Henry, Damian Morris, Gerry Rayman, Duncan Fowler, Susan Mitchell, Josephine Rosier, Rosemary Temple, Jeremy Turner, Gioia Canciani, Niranjala Hewapathirana, Leanne Piper, Ruth McManus, Anne Kudirka, Margaret Watson, Matteo Bonomo, Basilio Pintaudi, Federico Bertuzzi, Giuseppina Daniela Corica, Elena Mion, Julia Lowe, Ilana Halperin, Anna Rogowsky, Sapida Adib, Robert Lindsay, David Carty, Isobel Crawford, Fiona Mackenzie, Therese McSorley, John Booth, Natalia McInnes, Ada Smith, Irene Stanton, Tracy Tazzeo, John Weisnagel, Peter Mansell, Nia Jones, Gayna Babington, Dawn Spick, Malcolm MacDougall, Sharon Chilton, Terri Cutts, Michelle Perkins, Eleanor Scott, Del Endersby, Anna Dover, Frances Dougherty, Susan Johnston, Simon Heller, Peter Novodorsky, Sue Hudson, Chloe Nisbet, Thomas Ransom, Jill Coolen, Darlene Baxendale, Richard Holt, Jane Forbes, Nicki Martin, Fiona Walbridge, Fidelma Dunne, Sharon Conway, Aoife Egan, Collette Kirwin, Michael Maresh, Gretta Kearney, Juliet Morris, Susan Quinn, Rudy Bilous, Rasha Mukhtar, Ariane Godbout, Sylvie Daigle, Alexandra Lubina Solomon, Margaret Jackson, Emma Paul, Julie Taylor, Robyn Houlden, Adriana Breen, Anita Banerjee, Anna Brackenridge, Annette Briley, Anna Reid, Claire Singh, Jill Newstead Angel, Janet Baxter, Sam Philip, Martyna Chlost, Lynne Murray, Kristin Castorino, Lois Jovanovic, Donna Frase, Sonya Mergler, Kathryn Mangoff, Johanna Sanchez, Gail Klein, Katrina Ruedy, Craig Kollman, Olivia Lou, and Marlon Pragnell
Collaborators: On Behalf of the CONCEPTT Collaborative Group
Authors' Contributions
D.T. analyzed and interpreted the data and wrote the article. C.L.M., J.Y., and C.M.-B. contributed to the analysis, interpretation, and discussion of the data. I.G. contributed to the statistical analysis and interpretation of the data. D.S.F. contributed to the interpretation and discussion of the data. H.R.M. identified the study question and contributed to the interpretation and discussion of the data. R.C. designed the study, analyzed and interpreted the data, and revised the article. All authors reviewed the final version of the article before publication.
Author Disclosure Statement
D.T., C.L.M., J.Y., C.M.-B., and I.G. have no relevant conflicts of interest to report. D.S.F. has received honoraria for speaking engagements from Medtronic and has been on an advisory board for Novo Nordisk. H.R.M. has received honoraria for speaking engagements from Medtronic, Roche, Novo Nordisk, and Eli Lilly and is a member of the Medtronic European Advisory Board. R.C. has received honoraria for speaking engagements with Eli Lilly and Novo Nordisk and has been on an advisory boards for Novo Nordisk and Abbott.
Funding Information
The trial was funded by Juvenile Diabetes Research Foundation (JDRF) grants #17-2011-533, and grants under the JDRF Canadian Clinical Trial Network, a public–private partnership including JDRF and FedDev Ontario and supported by JDRF #80-2010-585. Medtronic supplied the CGM sensors and CGM systems at reduced cost. The subanalysis was funded by the EFSD/Sanofi European Pilot Research Grants for Innovative Measurement of Diabetes Outcomes, 2017. H.R.M. conducts independent research supported by the National Institute for Health Research (Career Development Fellowship, CDF-2013-06-035), and is supported by Tommy's charity. C.L.M. is supported by the Diabetes UK Harry Keen Intermediate Clinical Fellowship (DUK-HKF 17/0005712) and the EFSD-Novo Nordisk Foundation Future Leader's Award (NNF19SA058974).
Supplementary Material
Dr Lois Jovanovic died on September 18, 2018.
References
- 1. American Diabetes Association: 6. Glycemic targets: Standards of medical care in diabetes-2021. Diabetes Care 2021;44:S73–S84. [DOI] [PubMed] [Google Scholar]
- 2. Battelino T, Danne T, Bergenstal RM, et al. : Clinical targets for continuous glucose monitoring data interpretation: recommendations from the international consensus on time in range. Diabetes Care 2019;42:1593–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Feig DS, Donovan LE, Corcoy R, et al. : Continuous glucose monitoring in pregnant women with type 1 diabetes (CONCEPTT): a multicentre international randomised controlled trial. Lancet 2017;390:2347–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kristensen K, Ögge LE, Sengpiel V, et al. : Continuous glucose monitoring in pregnant women with type 1 diabetes: an observational cohort study of 186 pregnancies. Diabetologia 2019;62:1143–1153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Murphy HR: Continuous glucose monitoring targets in type 1 diabetes pregnancy: every 5% time in range matters. Diabetologia 2019;62:1123–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. American Diabetes Association: 14. Management of diabetes in pregnancy: Standards of medical care in diabetes-2021. Diabetes Care 2021;44:S200–S210. [DOI] [PubMed] [Google Scholar]
- 7. Bergenstal RM, Gal RL, Connor CG, et al. : Racial differences in the relationship of glucose concentrations and hemoglobin A1c levels. Ann Intern Med 2017;167:95–102. [DOI] [PubMed] [Google Scholar]
- 8. Lorenzo-Medina M, Uranga B, Rus A, et al. : Sex and age affect agreement between fasting plasma glucose and glycosylated hemoglobin for diagnosis of dysglycemia. Endocrinol Diabetes Nutr 2017;64:345–354. [DOI] [PubMed] [Google Scholar]
- 9. Nielsen LR, Ekbom P, Damm P, et al. : HbA1c levels are significantly lower in early and late pregnancy. Diabetes Care 2004;27:1200–1201. [DOI] [PubMed] [Google Scholar]
- 10. Kerssen A, Evers IM, De Valk HW, et al. : Poor glucose control in women with type 1 diabetes mellitus and ‘safe’ hemoglobin A1c values in the first trimester of pregnancy. J Matern Neonatal Med 2003;13:309–313. [DOI] [PubMed] [Google Scholar]
- 11. Law GR, Gilthorpe MS, Secher AL, et al. : Translating HbA1c measurements into estimated average glucose values in pregnant women with diabetes. Diabetologia 2017;60:618–624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Diabetes in pregnancy: management from preconception to the postnatal period. London: National Institute for Health and Care Excellence (UK); 2020 Dec 16. [PubMed] [Google Scholar]
- 13. Meek CL, Tundidor D, Feig DS, et al. : Novel biochemical markers of glycemia to predict pregnancy outcomes in women with type 1 diabetes. Diabetes Care 2021;44:681–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Feig DS, Asztalos E, Corcoy R, et al. : CONCEPTT: continuous glucose monitoring in women with type 1 diabetes in pregnancy trial: a multi-center, multi-national, randomized controlled trial—study protocol. BMC Pregnancy Childbirth 2016;16:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Porter H, Lookinland S, Belfort MA: Evaluation of a new real-time blood continuous glucose monitoring system in pregnant women without gestational diabetes: a pilot study. J Perinat Neonatal Nurs 2004;18:93–102. [DOI] [PubMed] [Google Scholar]
- 16. Yogev Y, Ben-Haroush A, Chen R, et al. : Diurnal glycemic profile in obese and normal weight nondiabetic pregnant women. Am J Obstet Gynecol 2004;191:949–953. [DOI] [PubMed] [Google Scholar]
- 17. Beck RW, Connor CG, Mullen DM, et al. : The fallacy of average: How using HbA1c alone to assess glycemic control can be misleading. Diabetes Care 2017;40:994–999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Law GR, Ellison GTH, Secher AL, et al. : Analysis of continuous glucose monitoring in pregnant women with diabetes: Distinct temporal patterns of glucose associated with large-for-gestational-age infants. Diabetes Care 2015;38:1319–1325. [DOI] [PubMed] [Google Scholar]
- 19. Abell SK, Boyle JA, de Courten B, et al. : Contemporary type 1 diabetes pregnancy outcomes: impact of obesity and glycaemic control. Med J Aust 2016;205:162–167. [DOI] [PubMed] [Google Scholar]
- 20. Ladfors L, Shaat N, Wiberg N, et al. : Fetal overgrowth in women with type 1 and type 2 diabetes mellitus. PLoS One 2017;12:e0187917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McGrath RT, Glastras SJ, Seeho SK, et al. : Association between glycemic variability, HbA1c, and large-for-gestational-age neonates in women with type 1 diabetes. Diabetes Care 2017;40:e98–e100. [DOI] [PubMed] [Google Scholar]
- 22. Stewart ZA, Wilinska ME, Hartnell S, et al. : Closed-loop insulin delivery during pregnancy in women with type 1 diabetes. N Engl J Med 2016;375:644–654. [DOI] [PubMed] [Google Scholar]
- 23. Polsky S: Pregnancy intervention with a closed-loop system (PICLS) Study (PICLS). Identification No. NCT03774186. https://clinicaltrials.gov/ct2/show/NCT03774186?term=NCT03774186&draw=2&rank=1 (accessed December 13, 2020).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.