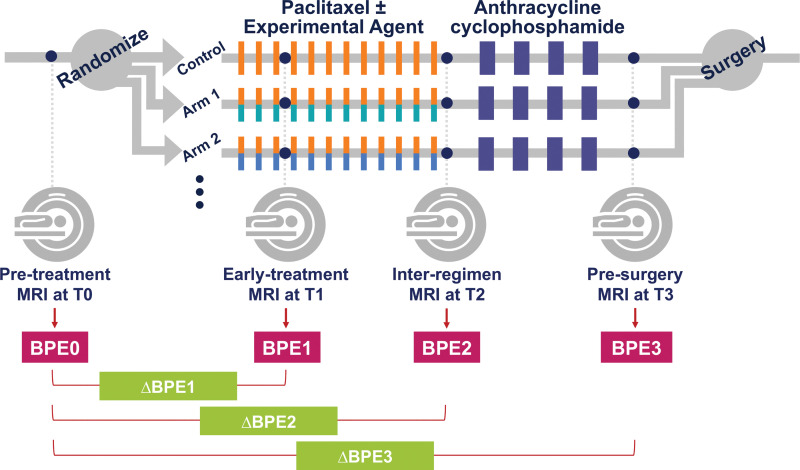
Figure 1:
Study schema. Patients were randomized to one of 10 drug arms (nine experimental drug arms and a standard of care control arm). An experimental agent or combination may substitute for part of the standard therapy (paclitaxel). Each patient underwent MRI examinations at four points during neoadjuvant chemotherapy. BPE0 = background parenchymal enhancement at T0, BPE1 = background parenchymal enhancement at T1, BPE2 = background parenchymal enhancement at T2, BPE3 = background parenchymal enhancement at T3, ΔBPE1 = percent change of background parenchymal enhancement relative to T0 at T1, ΔBPE2 = percent change of background parenchymal enhancement relative to T0 at T2, ΔBPE3 = percent change of background parenchymal enhancement relative to T0 at T3.