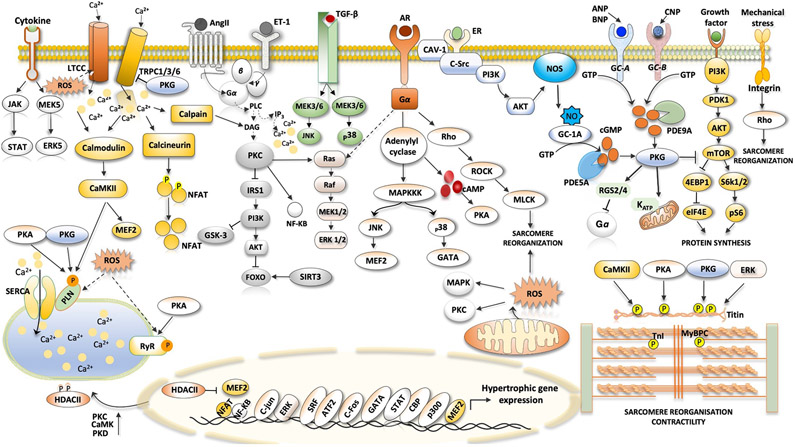
Fig. 1 ∣. Signalling pathways in cardiac hypertrophy.
Ventricular hypertrophy in heart failure with preserved ejection fraction, particularly in association with hypertension and neurohormonal stress, can involve many pathways identified in other hypertrophic syndromes. The figure shows the major pathways in cardiomyocytes that are thought to stimulate pathological muscle growth of the heart. The hormones angiotensin II (AngII), endothelin 1 (ET-1) and catecholamines bind to their cognate receptors, which are coupled to heterotrimeric G proteins to activate downstream signalling, such as the phospholipase C (PLC)–protein kinase C (PKC) axis. Activated PKC inhibits the insulin receptor substrate 1 (IRS1)–RACα serine/threonine-protein kinase (AKT)–forkhead box protein (FOXO) signalling pathway. β1-Adrenergic receptor (AR) stimulated protein kinase A (PKA) raises cytosolic Ca2+ levels by phosphorylation of Ca2+-handling proteins. Transient receptor potential channel 1 (TRPC1), TRPC3 and TRPC6 have been linked to pathological hypertrophy through elevated NFAT signalling. TRPC1 and TRPC6 are also mechanosensitive. Integrin transmembrane receptors also transduce intracellular hypertrophic signalling by activating downstream effectors such as Rho. Transforming growth factor-β (TGFβ) signalling and receptors transmitting signals through Gq-protein-coupled receptors promote activation of Rho-associated protein kinase (ROCK), extracellular-signal-regulated kinase (ERK) and p38 mitogen-activated protein kinase (MAPK), which contributes to pathological cardiac hypertrophy and fibrosis. Growth factor-mediated stimulation of mechanistic target of rapamycin (mTOR) signalling is linked to induction of protein synthesis and inhibition of autophagy. Hypertrophy is also associated with depressed cGMP–protein kinase G (PKG) signalling (both nitric oxide (NO)-mediated and natriuretic-peptide-mediated) and increased phosphodiesterase type 5A (PDE5A) and PDE9A expression. Many of these kinases can affect sarcomeric proteins, altering myofilament Ca2+ sensitivity and passive stiffness. Cytokines augment cardiac hypertrophy through their receptors (such as the IL-6 receptor). 4EBP1, eukaryotic translation initiation factor 4E-binding protein 1; AC, adenylyl cyclase; ANP, atrial natriuretic peptide; AT1R, angiotensin II receptor type 1; ATF2, cAMP-dependent transcription factor ATF2; BNP, B-type natriuretic peptide; CAMKII, Ca2+/calmodulin-dependent protein kinase II; CAV1, caveolin 1; CBP, CREB-binding protein; CNP, C-type natriuretic peptide; cTnI, cardiac troponin I; DAG, diacylglycerol; eIF4E, eukaryotic translation initiation factor 4E; ER, oestrogen receptor; FOS, proto-oncogene c-Fos; GATA4, transcription factor GATA4; HDAC, histone deacetylase; IP3, inositol trisphosphate; JAK, Janus kinase; JNK, JUN N-terminal kinase; JUN, proto-oncogene c-Jun; KATP, ATP-dependent K+ channel; LTCC, L-type Ca2+ channel; MAPKKK, mitogen-activated protein kinase kinase kinase; MEF2, myocyte enhancer factor 2; MEK, MAPK/ERK kinase; MLCK, myosin light chain kinase; MYBPC, cardiac myosin-binding protein C; NFAT, nuclear factor of activated T cells; NF-κB, nuclear factor-κB; NOS3, endothelial nitric oxide synthase; NPR-A, natriuretic peptide receptor A; NPR-B, natriuretic peptide receptor B; P, phosphate; p300, histone acetyltransferase p300; PDK1, 3-phosphoinositide-dependent protein kinase 1; PI3K, phosphoinositide 3-kinase; PKD, protein kinase D; PLN, cardiac phospholamban; pS6, ribosomal protein S6 kinase; RAF, RAF proto-oncogene serine/threonine-protein kinase; RGS2, regulator of G-protein signalling 2; RGS4, regulator of G-protein signalling 4; ROS, reactive oxygen species; RYR2, ryanodine receptor 2; S6K1, ribosomal protein S6 kinase-β1; S6K2, ribosomal protein S6 kinase-β2; SERCA2A, sarcoplasmic/endoplasmic reticulum Ca2+ ATPase; sGC, soluble guanylyl cyclase; SIRT3, mitochondrial NAD-dependent protein deacetylase sirtuin 3; SR, sarcoplasmic reticulum; SRC, proto-oncogene tyrosine-protein kinase SRC; SRF, serum response factor; STAT, signal transducer and activator of transcription; TGFBR, transforming growth factor-β receptor.