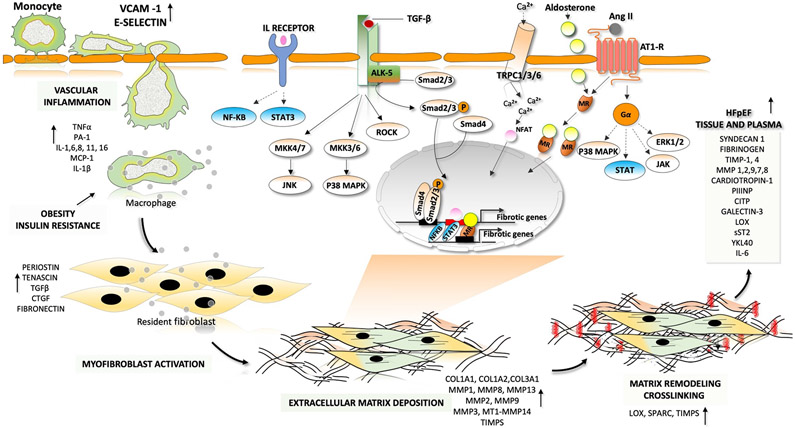
Fig. 2 ∣. Fibrotic–inflammatory remodelling in heart failure with preserved ejection fraction.
In patients with heart failure with preserved ejection fraction, endothelial cells produce factors that induce inflammation and recruit monocytes for transendothelial migration. These factors include interleukins (IL-1, IL-6 and IL-8), colony-stimulating factors (granulocyte-colony stimulating factor (G-CSF), macrophage colony-stimulating factor (M-CSF) and granulocyte-macrophage colony-stimulating factor (GM-CSF) and chemotactic factors (C-C motif chemokine 2 (CCL2)). Pro-inflammatory cytokines such as tumour necrosis factor (TNF), IL-1 and IL-6 are involved in the initiation and propagation of inflammatory signals. This inflammatory signalling stimulates monocytes and endothelial cells to initiate weak interactions and the subsequent rolling of the monocytes over the endothelial cells, mediated by adhesion molecules (such as vascular cell adhesion protein 1 (VCAM1) and E-selectin). Finally, monocytes migrate through the intercellular clefts between the endothelial cells to the underlying tissue. Macrophage-derived mediators and pro-inflammatory cytokines drive the transformation of quiescent fibroblasts into proliferative and matrix-synthesizing active myofibroblasts. The communication between inflammatory cells and resident fibroblasts occurs through direct cell–cell interactions and through paracrine signalling. Sustained activation of myofibroblasts produces structural extracellular matrix (ECM) proteins and matricellular proteins. Crosslinking of collagen molecules by matrix-crosslinking enzymes, such as lysyl oxidase (LOX), prevents the enzymatic degradation of collagen, leading to an increase in collagen content and stiffness. The dynamic alterations in the composition of the ECM and the prolonged deposition of ECM modulates cardiac function. Both canonical transforming growth factor-β (TGFβ)– mothers against decapentaplegic homologue 2 (SMAD2)–SMAD3 signalling and non-canonical TGFβ signalling through Rho-associated protein kinase (ROCK), extracellular-signal-regulated kinase (ERK) and p38 contribute to the development of pathological hypertrophy and fibrosis. Phosphorylation and activation of ERK, p38, Janus kinase (JAK) and signal transducer and activator of transcription (STAT) by activation of G-protein-coupled receptors also leads to the development of fibrosis. Activation of the calcineurin–nuclear factor of activated T cells (NFAT) pathway by cardiac transient receptor potential channels (TRPCs) also has a major role in fibrotic remodelling. Mineralocorticoid receptors (MRs) are ligand-activated transcription factors that induce transcription of profibrotic genes upon aldosterone binding. AngII, angiotensin II; AT1R, angiotensin II receptor type 1; CITP, carboxy-terminal propeptide of procollagen type I; COL, collagen; CTGF, connective tissue growth factor; JNK, JUN N-terminal kinase; MAPK, mitogen-activated protein kinase; MMP, matrix metalloproteinase; MEK, MAPK/ERK kinase; NF-κB, nuclear factor-κB; P, phosphate; PIIINP, amino-terminal propeptide of procollagen type III; sST2, soluble protein ST2; TGFBR1, transforming growth factor-β receptor type 1; TIMP, metalloproteinase inhibitor; YKL40, chitinase-3-like protein 1.